Written by Pendell Meyers
Two adult patients in their 50s called EMS for acute chest pain that started within the last hour. Both were awake and alert with normal vital signs. Both cases had an EMS ECG that was transmitted to the ED physician asking "should we activate the cath lab?"
What do you think? Here they are:
Patient 1, ECG1:
 |
| Zoll computer algorithm stated: "***STEMI***, Anterior Infarct" |
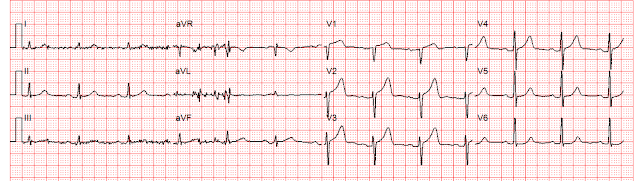 |
| Zoll computer algorithm stated: "ST elevation, probably benign early repolarization..." |
Queen of hearts interpretations:
Patient 1, ECG1:
Patient 2, ECG1:
Patient 1 Clinical Course and Outcome:
The EM physician did not see that the S wave voltage has been truncated and squared off at 10 mm, thereby greatly limiting the assessment of proportionality. He diagnosed anterior "STEMI" and activated the cath lab.
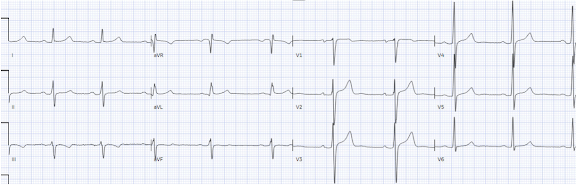
On arrival to the ED, while waiting for cath lab team, he obtained another ECG:
Precordial Swirl -- 20 cases of Swirl or Look-Alikes
Patient 2 Clinical Course and Outcome:
 |
| Post-PCI. |
 |
| Reperfusion pattern. |
MY Comment, by KEN GRAUER, MD (11/29/2023):
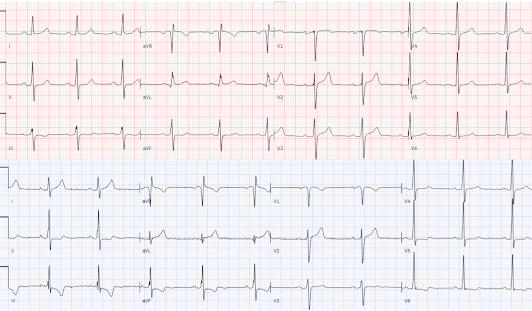
- For clarity in Figure-1 — I’ve reproduced and labeled the initial EMS Tracings for the 2 patients in today’s case.
- To Emphasize: Both of these patients presented with new-onset CP (Chest Pain).
- My Initial Thoughts: In view of the history of new CP — I fully acknowledge that I initially found it difficult to decide if one (or both) of these patients were in process of evolving an acute OMI.
- Once aware of this automatic truncation of ECG amplitudes in most EMS systems in the United States — it becomes easy to spot. The dotted BLUE lines in the initial EMS tracing for Patient #1 — show truncation of the S waves in leads V2,V3 and V4 — and truncation of the R wave in leads V5,V6. (I put ?? for the S wave in lead V1 — because I wasn't certain if the S wave in this lead was precisely 10 mm, or perhaps slightly truncated).
- The greater the distance between the ascending/descending arms of the R wave or S wave at the 10 mm cutoff point — the larger the actual amplitude of the S wave or R wave is likely to be. For example, in Patient #1 — I suspect that the largest actual S wave will be in lead V3, given the large opening at the 10 mm cutoff.
- The "truncation effect" — is clearly less significant in the initial EMS tracing for Patient #2 — being present only in leads V2,V3 (and most probably not marked given virtual closure of ascending-descending arms of the S wave at the 10 mm cutoff in these leads).
- As per Dr. Meyers — significant truncation of either S wave or R wave amplitude may markedly distort ST-T wave appearance, rendering the important concept of "proportionality" as useless. And, as was demonstrated in today's Case for Patient #1 — the repeat ECG in the ED revealed that the reason for truncation was the presence of greatly increased QRS amplitudes, as a result of marked LVH (and in the context of LVH — the elevated anterior ST-T waves seen in the initial EMS tracing for Patient #1 were consistent with the appearance of LV "strain" in anterior leads).
- PEARL #1: It's important to be aware of this automatic truncation effect so commonly seen in EMS ECGs — because one might otherwise misinterpret the seemingly "large" ST-T waves as disproportionate to a short S wave, and therefore presume this to represent a hyperacute T wave (Please see My Comment at the bottom of the page in the June 20, 2020 — the February 6, 2020 — and November 14, 2023 posts in Dr. Smith's ECG Blog).
- PEARL #2: The automatic EMS truncation effect is most likely to cause confusion in chest pain patients who manifest marked LVH with deep anterior S waves (just as we saw for Patient #1 in today's case — and as I describe in the December 27, 2018 post, as well as in the above posts that I cited in Pearl #1).
- PEARL #3: As per Dr. Meyers — The 3rd KEY concept to be aware of in the interpretation of EMS tracings of CP patients with LVH — is "Precordial Swirl". The October 15, 2022 post in Dr. Smith's ECG Blog features no less than 20 ECG examples by Drs. Meyers and Smith of what is and what is not "Precordial Swirl" from proximal LAD occlusion (In My Comment at the bottom of the page in this Oct. 15 post — I consolidate KEY features to facilitate identification of this Precordial Swirl Pattern).
- For Patient #1: I instantly recognized truncation of the S wave in leads V2,V3,V4 (and possibly also in lead V1) — as well as truncation of R wave height in leads V5,V6. Especially in view of the wide opening between descending and ascending arms of the S wave in lead V3 — I was virtually certain there was marked LVH — and, that this might account for all of the ST-T wave findings seen in this initial EMS tracing.
- The above said — I was not 100% certain that LVH explained everything in the initial EMS ECG from this middle-aged adult with new CP. I thought the peak of the T wave in lead V2 seemed "fatter"-than-expected — the base of the T wave in lead V3 seemed "wider"-than-expected — the J-point depression in lead V6 was with a flattend ST segment (not typical for LV "strain") — and there was subtle ST flattening in leads III and aVF — with ST coving and shallow T wave inversion in lead aVL.
- My BOTTOM Line for Patient #1: I would not have activated the cath lab on the basis of this initial tracing. I suspected LVH as the main issue here — but felt the need for additional evaluation, including a look at the initial ED ECG to better appreciate the true proportionality of ST-T waves. And as shown by Dr. Meyers in his above discussion — the repeat ECG in the ED without truncation — strongly supported the premise that abnormal findings were the result of marked LVH and not acute OMI.
- For Patient #2: I again instantly recognized truncation of the S wave in leads V2 and V3 — albeit lack of significant "opening" between descending and ascending arms of the S wave in these leads suggested that S wave depth was less likely to be deep enough to account for the surprisingly tall T waves, with "fat" peak and wide base in these leads.
- KEY Point: There is no truncation in lead V1 of the initial Patient #2 EMS tracing! (BLUE arrows in these leads showing S wave amplitude clearly below the 10 mm cutoff point!). Given that there is no truncation in lead V1 — there is NO WAY that the straightening of the elevated ST segment takeoff, with disproportionately tall and "fat" T wave (considering the tiny amplitude of the S in V1) could possibly be normal. In this patient with new CP — acute LAD OMI has to be assumed until proven otherwise.
- My BOTTOM Line for Patient #2: Once I realized that lead V1 (within the BLUE rectangle) was definitely abnormal — I became more convinced that the T waves in neighboring leads V2,V3,V4 were hyperacute until proven otherwise. It was no surprise to learn that this patient evolved total mid-LAD occlusion.











-USE.png)









-USE.png)