A male in his 20's with no past medical history was found in cardiac arrest. He had been complaining of chest pain. Medics found him in ventricular fibrillation. It is uncertain whether he had bystander CPR. After one shock, he was in PEA, then VF again. He had multiple doses of epinephrine and also amiodarone.
Of course he was undergoing chest compressions the entire time. He had a King airway in place.
On arrival to the ED, approximately 50 minutes after arrest, he was still in full arrest. A rhythm check showed asystole. Chest compressions were continued with the LUCAS device. He was intubated. The ResQPod (inspiratory threshold device) was applied to the endotracheal tube to increase venous return and cardiac output. He was given more epinephrine, bicarbonate, and calcium in a last ditch effort to resuscitate him. End-tidal CO2 was 18-20, then improved to 30-40.
A rhythm check revealed a slow regular wide complex rhythm with ST elevation apparent on the monitor. A cardiac ultrasound revealed no significant motion. There was only a "flicker" of movement with each electrical complex.
More Epi, bicarb, and atropine was given. SpO2 was 80%.
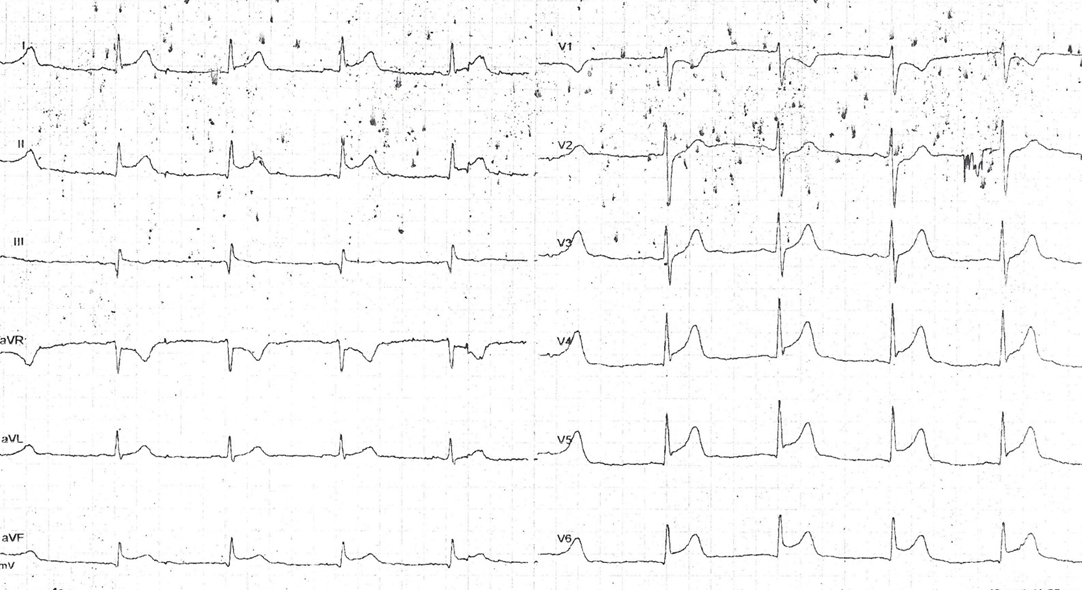
A 12-lead ECG was recorded at approximately 70 minutes after arrest:
[RBBB and LAFB, with STE, are associated with massive MI from left main or LAD occlusion. See this incredible case. And also this amazing case of resuscitation after 68 minutes of CPR.]
Case continued:
Chest Compressions were continued. The interventionalist was consulted. The cath lab was activated and the interventionalist also recommended tPA until cath could be done (it was early AM, the team was not in-house, and angiogram/PCI would be delayed). Bolus and infusion were started at about 80 minutes after arrest.
VBG at this time showed pH of 6.94, pCO2 72, bicarb 18 and 69% O2 saturation. Lactate was 11.3 mEq/L. Pulseox was mid 80s and etCO2 was 25 mmHg.
Esmolol was given to help prevent further VF. (See abstract below: esmolol may be beneficial for refractory VF. I would be worried that it would be detrimental in PEA.)
The patient was transported to the cath lab at approximately 120 minutes after arrest, with LUCAS CPR ongoing.
Outcome:
Angiography showed occlusion of the LAD and it was opened and stented with a bare metal stent. However, the patient never regained cardiac function.
Angiography and PCI during CPR
In this study from 2010, 11 of 36 patients who underwent PCI during LUCAS chest compressions survived neurologicially intact.
In this study from 2007, PCI was successful in all 11 patients, but only 3 of 11 survived the procedure and none of them were discharged alive.
Here is a nice summary of research on PCI during CPR, written by Ron Johannsen of Hennepin County Medical Center: They conclude:
"Currently available data do not support routine [my emphasis] activation of the catheterization laboratory for patients without ROSC, even if mechanical CPR is being performed. Whether use of the LUCAS™ or other devices will affect outcome is uncertain; we will have to await the completion of ongoing clinical trials. For patients with cardiac arrest in the catheterization laboratory, however, it seems reasonable to institute mechanical CPR when this is available. Alternatively, other means of cardiac support, such as early transition of patients to ECMO or assist devices, to allow intra-arrest PCI could be mobilized for selected patients."
Who should be considered for PCI during chest compressions, in my opinion?
Fibrinolysis unlikely to be of benefit
tPA has not been shown effective for cardiac arrest, whether with PEA (suggesting pulmonary embolism) or otherwise. It does not even decrease mortality due to STEMI-related cardiogenic shock.
Utility of angiography and PCI in patients with ROSC:
Spaulding article on prediction of coronary etiology of arrest: http://www.nejm.org/doi/pdf/10.1056/NEJM199706053362302
84 patients resuscitated and had cardiac etiology, 60 patients with clinically significant CAD, 40 with occlusion, 9 of these had no ST elevation. The only independent predictors of occlusion were ST-segment elevation (odds ratio, 4.3; 95 percent confidence interval, 1.6 to 2; P 0.004) and chest pain before the arrest (odds ratio, 4.0; 95 percent confidence interval, 1.3 to 10.1; P 0.016). The presence of one of these two factors was associated with positive and negative predictive values of 0.63 and 0.74, respectively, and the presence of both with values of 0.87 and 0.61. How expert were the readers of these ECGs at finding ischemia?
Successful angioplasty was the best predictor of survival: the following factors were predictive of survival: absence of the need for inotropic drugs during transportation to the hospital (odds ratio, 3.6; 95 percent confidence interval, 1.1 to 11.8; P 0.03) and successful coronary angioplasty (odds ratio, 5.2; 95 percent confidence interval, 1.1 to 24.5; P 0.04).
In the study by Silfvast (1991), 78% of patients with ventricular fibrillation had "coronary disease" as the etiology.
Here is an outstanding powerpoint on the need for angiography and PCI after return of spontaneous circulation, written by Demetris Yannopoulos, who, along with Keith Lurie (here at Minneapolis Medical Research Foundation, associated with Hennepin County Medical Center and the University of Minnesota), is doing incredible bench research on cardiac arrest. Here is an EMCrit podcast on their spectacular advances.
Which patients with ROSC after out of hospital arrest need immediate angiography/PCI?
The case that Dr. Yannopoulos uses in his presentation to illustrate the "negative" ECG in a patient with arrest and LAD occlusion does, in fact, show ischemia: there is very suble STE in aVL and reciprocal ST depression in II, III, and aVF. Many would miss this. Very expert ECG readers would see it.
My guideline
Abstract on Esmolol, to be presented at Social Media and Critical Care 2014, Gold Coast, Australia:
Of course he was undergoing chest compressions the entire time. He had a King airway in place.
On arrival to the ED, approximately 50 minutes after arrest, he was still in full arrest. A rhythm check showed asystole. Chest compressions were continued with the LUCAS device. He was intubated. The ResQPod (inspiratory threshold device) was applied to the endotracheal tube to increase venous return and cardiac output. He was given more epinephrine, bicarbonate, and calcium in a last ditch effort to resuscitate him. End-tidal CO2 was 18-20, then improved to 30-40.
A rhythm check revealed a slow regular wide complex rhythm with ST elevation apparent on the monitor. A cardiac ultrasound revealed no significant motion. There was only a "flicker" of movement with each electrical complex.
More Epi, bicarb, and atropine was given. SpO2 was 80%.
A 12-lead ECG was recorded at approximately 70 minutes after arrest:
[RBBB and LAFB, with STE, are associated with massive MI from left main or LAD occlusion. See this incredible case. And also this amazing case of resuscitation after 68 minutes of CPR.]
Case continued:
Chest Compressions were continued. The interventionalist was consulted. The cath lab was activated and the interventionalist also recommended tPA until cath could be done (it was early AM, the team was not in-house, and angiogram/PCI would be delayed). Bolus and infusion were started at about 80 minutes after arrest.
VBG at this time showed pH of 6.94, pCO2 72, bicarb 18 and 69% O2 saturation. Lactate was 11.3 mEq/L. Pulseox was mid 80s and etCO2 was 25 mmHg.
Esmolol was given to help prevent further VF. (See abstract below: esmolol may be beneficial for refractory VF. I would be worried that it would be detrimental in PEA.)
The patient was transported to the cath lab at approximately 120 minutes after arrest, with LUCAS CPR ongoing.
Outcome:
Angiography showed occlusion of the LAD and it was opened and stented with a bare metal stent. However, the patient never regained cardiac function.
Angiography and PCI during CPR
In this study from 2010, 11 of 36 patients who underwent PCI during LUCAS chest compressions survived neurologicially intact.
In this study from 2007, PCI was successful in all 11 patients, but only 3 of 11 survived the procedure and none of them were discharged alive.
Here is a nice summary of research on PCI during CPR, written by Ron Johannsen of Hennepin County Medical Center: They conclude:
"Currently available data do not support routine [my emphasis] activation of the catheterization laboratory for patients without ROSC, even if mechanical CPR is being performed. Whether use of the LUCAS™ or other devices will affect outcome is uncertain; we will have to await the completion of ongoing clinical trials. For patients with cardiac arrest in the catheterization laboratory, however, it seems reasonable to institute mechanical CPR when this is available. Alternatively, other means of cardiac support, such as early transition of patients to ECMO or assist devices, to allow intra-arrest PCI could be mobilized for selected patients."
Who should be considered for PCI during chest compressions, in my opinion?
- Out of hospital arrest: Patients who:
- Have witnessed ventricular fibrillation arrest, especially if preceded by chest pain
- Receive bystander CP
- Are relatively young without comorbidities
- Are undergoing excellent CPR (e.g., LUCAS)
- Have short arrest to treatment times and can undergo immediate PCI.
- This case had PEA
- But ventricular fibrillation was the initial rhythm
- Although there was PEA, it was unusual in that the electrical activity was very recognizable and organized and clearly due to STEMI.
- The time from arrest to PCI was probably too long to expect a good outcome.
- ED arrest from STEMI/STEMI-equivalent: relatively young without comorbities, good compressions on LUCAS, or put on ECMO.
- Cath lab arrest
Fibrinolysis unlikely to be of benefit
tPA has not been shown effective for cardiac arrest, whether with PEA (suggesting pulmonary embolism) or otherwise. It does not even decrease mortality due to STEMI-related cardiogenic shock.
Utility of angiography and PCI in patients with ROSC:
Spaulding article on prediction of coronary etiology of arrest: http://www.nejm.org/doi/pdf/10.1056/NEJM199706053362302
84 patients resuscitated and had cardiac etiology, 60 patients with clinically significant CAD, 40 with occlusion, 9 of these had no ST elevation. The only independent predictors of occlusion were ST-segment elevation (odds ratio, 4.3; 95 percent confidence interval, 1.6 to 2; P 0.004) and chest pain before the arrest (odds ratio, 4.0; 95 percent confidence interval, 1.3 to 10.1; P 0.016). The presence of one of these two factors was associated with positive and negative predictive values of 0.63 and 0.74, respectively, and the presence of both with values of 0.87 and 0.61. How expert were the readers of these ECGs at finding ischemia?
Successful angioplasty was the best predictor of survival: the following factors were predictive of survival: absence of the need for inotropic drugs during transportation to the hospital (odds ratio, 3.6; 95 percent confidence interval, 1.1 to 11.8; P 0.03) and successful coronary angioplasty (odds ratio, 5.2; 95 percent confidence interval, 1.1 to 24.5; P 0.04).
In the study by Silfvast (1991), 78% of patients with ventricular fibrillation had "coronary disease" as the etiology.
Here is an outstanding powerpoint on the need for angiography and PCI after return of spontaneous circulation, written by Demetris Yannopoulos, who, along with Keith Lurie (here at Minneapolis Medical Research Foundation, associated with Hennepin County Medical Center and the University of Minnesota), is doing incredible bench research on cardiac arrest. Here is an EMCrit podcast on their spectacular advances.
Which patients with ROSC after out of hospital arrest need immediate angiography/PCI?
The case that Dr. Yannopoulos uses in his presentation to illustrate the "negative" ECG in a patient with arrest and LAD occlusion does, in fact, show ischemia: there is very suble STE in aVL and reciprocal ST depression in II, III, and aVF. Many would miss this. Very expert ECG readers would see it.
My guideline
- If another definite etiology of arrest is found, other than ischemia, angiography/PCI is not indicated.
- If no other etiology is found, then patients with any of the following should go to PCI:
- Preceded by chest pain
- Ventricular fibrillation with no known prior cardiomyopathy or channelopathy
- Any ischemia on the ECG, as interpreted by a very expert reader.
- Since most ECG interpreters are not very expert, then in most cases any patient without a known etiology should go to the cath lab
Abstract on Esmolol, to be presented at Social Media and Critical Care 2014, Gold Coast, Australia:
Emergency Department Use of Esmolol in Refractory Ventricular Fibrillation
OBJECTIVE
We describe the outcomes for patients receiving esmolol during refractory ventricular fibrillation (RVF) in the emergency department (ED).
METHODS
A structured chart review in an urban academic ED of patients between January 2011 and March 2013 who received esmolol with an ED diagnosis of cardiac arrest (CA), ventricular fibrillation, or pulseless ventricular tachycardia, excluding patients who received esmolol before CA or after sustained return of spontaneous circulation (ROSC). Cardiac rhythms, CA management, timing of ROSC, and patient outcomes were recorded.
RESULTS
Six male patients met inclusion criteria; one was excluded because esmolol was administered after sustained ROSC. Four of five patients had out-of-hospital CA; all had automatic mechanical chest compressions delivered by a LUCAS™ device. All patients received repeated doses of epinephrine, amiodarone, lidocaine, sodium bicarbonate, as well as other adjunctive medications. Defibrillation was attempted many times for each patient prior to esmolol administration (median = 6.5, range 4-10). Some had temporary ROSC, but no patient had sustained ROSC after administration of these medications and defibrillation. All patients had a rhythm of VF at the time of esmolol administration. An esmolol loading dose and infusion of 500 mcg/kg and 50-100 mcg/kg/min, respectively, was subsequently administered to all patients. One patient with incessant VF achieved temporary ROSC and three others attained sustained ROSC after the administration of esmolol with repeat defibrillation; two survived to discharge with excellent neurologic outcomes.
CONCLUSION
Beta-blockade should be considered in all patients with RVF in the ED prior to cessation of resuscitative efforts.
We describe the outcomes for patients receiving esmolol during refractory ventricular fibrillation (RVF) in the emergency department (ED).
METHODS
A structured chart review in an urban academic ED of patients between January 2011 and March 2013 who received esmolol with an ED diagnosis of cardiac arrest (CA), ventricular fibrillation, or pulseless ventricular tachycardia, excluding patients who received esmolol before CA or after sustained return of spontaneous circulation (ROSC). Cardiac rhythms, CA management, timing of ROSC, and patient outcomes were recorded.
RESULTS
Six male patients met inclusion criteria; one was excluded because esmolol was administered after sustained ROSC. Four of five patients had out-of-hospital CA; all had automatic mechanical chest compressions delivered by a LUCAS™ device. All patients received repeated doses of epinephrine, amiodarone, lidocaine, sodium bicarbonate, as well as other adjunctive medications. Defibrillation was attempted many times for each patient prior to esmolol administration (median = 6.5, range 4-10). Some had temporary ROSC, but no patient had sustained ROSC after administration of these medications and defibrillation. All patients had a rhythm of VF at the time of esmolol administration. An esmolol loading dose and infusion of 500 mcg/kg and 50-100 mcg/kg/min, respectively, was subsequently administered to all patients. One patient with incessant VF achieved temporary ROSC and three others attained sustained ROSC after the administration of esmolol with repeat defibrillation; two survived to discharge with excellent neurologic outcomes.
CONCLUSION
Beta-blockade should be considered in all patients with RVF in the ED prior to cessation of resuscitative efforts.