Submitted and written by Quinton Nannet, MD, peer reviewed by Meyers, Grauer, Smith
A woman in her 70s recently diagnosed with COVID was brought in by EMS after she experienced acute onset sharp midsternal chest pain without radiation or dyspnea. She felt nauseous and lightheaded with no neurologic deficits. EMS noted prehospital vitals for heart rates in the 60s, SPO2 of 98% on room air, initially hypotensive to 66/34 with improvement to 100/70 after 800 mL of IV fluids by EMS. Here is her ECG on arrival to the ED:
 |
| What is your differential? What are your next steps? |
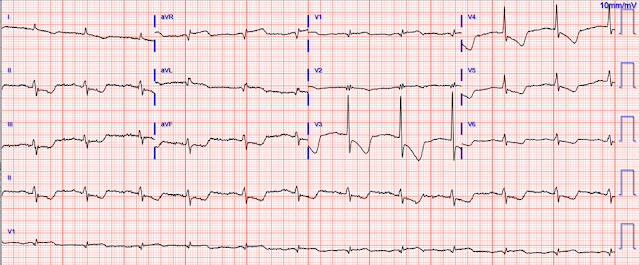
The ECG is quickly reviewed and shows sinus rhythm with normal QRS complexes. There is ST depression in leads V3-V6, I, aVL, II, III, and aVF, with ST elevation in aVR. Importantly, there is also STE in aVL, as well as V1. The differential is:
Posterolateral OMI or subendocardial ischemia
The distinction between posterior OMI and subendocardial ischemia can be important and sometimes difficult. Usually, ST depression proportionally maximal in V1-V4 indicates posterior OMI, whereas ST depression maximal in V5-V6 (with similar STD in II and reciprocal STE in aVR) indicates subendocardial ischemia. In posterior OMI, the STD in V4 improves significantly or resolves completely from V4 to V6; whereas, in subendocardial ischemia, the STD persists in severity or worsens from V4 to V6.
STD/R wave ratios:
V3: 2.5 / 16.5 = 15%
V4: 2.0 / 11.0 = 18%
V5: 0.5 / 5 = 10%, or perhaps it is closer to 0.75 / 5 = 15%
V6: 0.5 / 3 = 17%
So this is a difficult one, as the STD may be objectively maximal in V4, but still persists from V4 to V6. The STE in aVL should probably be considered lateral OMI until proven otherwise (thus also making posterior OMI more likely), but in subendocardial ischemia the leads with upward/superior components (aVR, V1, aVL) can all show reciprocal STE from the diffuse downward and leftward STD.
Her prior ECG on file is shown below:
What are your next steps?
Do you activate the Cath Lab?
Given her still undifferentiated hypotension, a RUSH exam was performed. It was notable for a normal cardiac ultrasound with no pericardial fluid, normal LV and RV function (though the quality was not sufficient to evaluate for wall motion abnormalities) and normal IVC dynamics. There was no free fluid noted in the thorax, right upper quadrant, left upper quadrant, or retrovesicular views. The probe was then moved to the aorta.
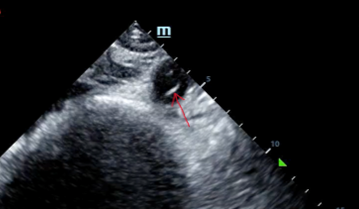
A dissection flap is noted in the intrabdominal aorta, and the aortic outflow tract is also noted to appear wider than normal. She was taken immediately for a CT angiogram of the chest, abdomen and pelvis.
Type A Axial.mov from Pendell Meyers on Vimeo.
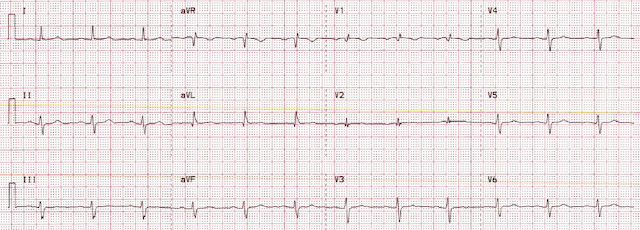
The CT angio showed a type A aortic dissection extending from the aortic root proximally to the carotid and left subclavian artery and distally to the common femoral arteries. Cardiothoracic and vascular surgery were consulted and the patient was taken to the OR within an hour and a half of her arrival to the ED. Intraoperative TEE noted "Type A aortic dissection arising 1.0 cm distal to the non-coronary cusp of the aortic valve." The operative report and radiology reads do not comment specifically as to whether the dissection flap was partially or fully obstructing coronary flow, or whether it was obstructing the left main or the RCA. She was noted to have a blood-tinged pericardial effusion. Her dissection was repaired and her aortic valve was resuspended. On postop day 1 she was extubated and the following ECG was obtained:
 |
| Resolution of STD, and no obvious posterolateral reperfusion findings. |
Her initial ED high sensitivity troponin was less than 6ng/L (below limit of detection), and none further were ordered.
Intraoperative TEE (before intervention) showed:
Normal LV size and thickness
Normal systolic function with EF 60%
No segmental wall motion abnormalities
Normal RV function
Aortic dissection false lumen prolapses into the aortic valve creating severe aortic valve insufficiency, 3-4+ aortic regurgitation
The patient progressed well throughout her hospital stay and was discharged on post-op day 6. Wonderfully, she has returned to her normal life with no significant co-morbidities from her dissection.
Learning Points:
The ECG is always just one piece of the clinical puzzle. Bedside ultrasound is another very important piece. It worthwhile to perform ultrasound on all chest pain patients, but particularly if there are any hemodynamic signs.
Ischemic ST depression includes posterior OMI and subendocardial ischemia. In the context of ACS, ST depression maximal in V1-4 has been shown to be fairly specific for posterior OMI. Usually the STD of posterior OMI improves and resolves from V4 to V6, whereas subendocardial ischemia STD typically persists or worsens from V4 to V6.
STD maximal in V5-6 and lead II, with reciprocal STE in aVR, typically indicates global supply/demand mismatch subendocardial ischemia, which can be due to a huge variety of clinical causes, including life-threatening ACS.
Ultrasound can be very helpful to distinguish causes of hypotension. Sometimes a dissection flap can be seen using bedside ultrasound.
Further Reading:
http://hqmeded-ecg.blogspot.com/2012/02/five-primary-patterns-of-ischemic-st.html
See these relevant cases:
A man in his 50s with acute chest pain and diffuse ST depression
- To Emphasize: The presentation by Drs. Nannet and Meyers is complete and superb. It clearly covers all KEY educational points.
- My initial impression was that something must be "off". But nothing was off — and lead placement was correct (verified by nearly-identical looking leads V1 and V2 on the prior tracing in the chart).
- The other "eye-catching" lead in ECG #1 was lead V4. While not quite as large as the complex in lead V3 — QRS amplitude, and ST-T wave size was clearly much greater than that seen in the other 10 leads.
- That said, as per Drs. Nannet and Meyers — there is ST-T wave depression in 8/12 leads, with the relative amount of ST-T wave deviation being comparable in each of these 8 leads when one considers the relative differences in QRS amplitudes!
- In the remaining leads — there is worrisome ST elevation in aVR, aVL and V1 — with a hint of ST coving (if not slight elevation) in lead V2.
- BUT — If I had not been given this history — My differential diagnosis on seeing this tracing would clearly be expanded. In addition to the 2 principal considerations put forth by Drs. Nannet and Meyers ( = acute posterolateral OMI and diffuse subendocardial ischemia) — I would add myocarditis (since the patient has newly diagnosed COVID) — acute PE (since there is worrisome-looking ST-T depression in the inferior and mid-chest leads) — and some form of multi-vessel coronary disease (given how hard-pressed I felt trying to explain all the ST-T wave depression in the face of worrisome-looking ST elevation in leads aVL and V1).
- To the above (especially given this patient's age) — I'd add Takotsubo cardiomyopathy, given at least slight QTc prolongation and the unusual distribution and magnitude of ST-T wave deviations.
- And — I wondered if the net positive tiny QRS in lead V1 represent a new or old finding related to incomplete RBBB vs posterior infarction.
- Credit to the treating clinicians — as this case was solved in large part due to the tip-off provided by bedside ultrasound that did not stop with the cardiac exam, but which continued to assess the aorta.
- PEARL: We think of diffuse subendocardial ischemia as being characterized by ST depression in multiple leads with ST elevation in lead aVR. But as per Drs. Nannet and Meyers — ST elevation may also sometimes be seen in leads aVL and V1 with this entity!
- The patient made a complete recovery thanks to quick actions by the treating clinicians — with successful surgical repair of her aortic dissection.
- In retrospect — all ECG findings in the "eye-catching" initial tracing were explained. Diffuse ST-T wave depression on the initial tracing was the result of shock from the patient's aortic dissection. Considering reduced QRS amplitudes — the relative amount of ST-T wave depression was comparable in the 8 leads that showed this finding. ST elevation in leads aVR, aVL and V1 was part of this ECG presentation of diffuse subendocardial ischemia. The tiny positive QRS complex in lead V1 was longstanding. And following surgical repair — ST-T wave changes for the most part resolved — and the final ECG was no longer "eye-catching"!
-USE%20copy.png) |
| Figure-1: I've reproduced the initial ECG in today's case. |


No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.