This was written by Brooks Walsh @BrooksWalsh, an emergency physician in Connecticut.
A paced ECG
The family of a very elderly person called EMS when they became short of breath. The patient had a number of comorbidities, including a pacemaker.
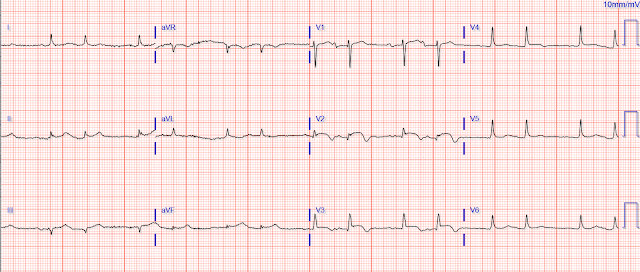
EMS obtained a number of ECGs, including this one:
Could a cath lab activation be justified with this ECG?
Well, yes, it should be!
The classic- and modified-Sgarbossa criteria for determining acute MI in the context of a paced rhythm are likely already well appreciated by readers of this blog. This ECG is a great illustration of those rules, particularly the criterion that ST elevation that exceeds 20-25% of the depth of the S wave (“excessive discordance”) suggests acute coronary occlusion.
We see this in lead V3, where the ST segment in the first QRS complex is about 3.6 mm high, and the S wave 13.7 mm deep - this gives a ratio of 0.26. There is some unfortunate baseline wander, but the proportion holds for the 3rd complex as well. (The second complex is uninterpretable, as the S wave runs off the paper).
Lead III shows similar excessive discordance, with the third beat giving a ratio of 3.5/11.7, or 0.29. The fourth beat (3.5/10.4 = 0.33) is distorted but diagnostic.
Additionally, the ST segment in V2 suggests > 1 mm of concordant STE (in beats #2 and #3), but the baseline wander here makes this less certain.
So, did these rules correctly predict a coronary occlusion?
Although angiography was not performed in this case, there is enough evidence to reasonably “prove” acute coronary occlusion. First of all, the paramedics had obtained another ECG about 10 minutes earlier:This non-paced ECG shows ST elevations in the inferior and anterolateral leads, as well as ST depression in aVL, suggesting a “wrap-around” LAD lesion.The non-paced ECG subsequently obtained in the ED was similar:
This was markedly different from an ECG obtained 1 year prior:
After evaluation by the emergency physician and the interventionist, and a discussion with the patient and family, neither angiography nor chemical fibrinolysis were pursued (the patient was quite old and debilitated).
Although angiography was not performed, acute occlusion of the LAD was supported by other tests. The initial troponin was 2.3 ng/ml (< 0.01), rising to 4 ng/ml about 10 hours later. The patient became hypotensive and showed signs of CHF. An echocardiogram showed severe systolic dysfunction, with akinesis of the apex, mid anterior, mid anterolateral, mid inferoseptal, mid anteroseptal, and mid inferolateral segments (Prior echo had been normal for age).
 |
| Apical 4 chamber in systole |
Lastly, an ECG obtained the next day showed evolving lateral T waves, further supporting an acute occlusion.
The PERFECT Study [From the Paced ECG Requiring Fast Emergent Coronary Therapy (PERFECT) Study Group]
The PERFECT trial will show that acute coronary occlusion can be reliably predicted from paced ECGs, despite the prevailing belief that paced ECGs are “uninterpretable.” That trial used angiographic data, so this case (despite good circumstantial evidence) would not have met inclusion criteria. Hopefully the rigorous methodology can change the out-dated perspectives of emergency physicians and cardiologists!
The study is now under revision for Annals of Emergency Medicine.
Below is the Results and Conclusion portion of the abstract, which was published in AEM and presented at SAEM in 2018. Annals is requesting that we also look at patients with AMI but without occlusion (Non-OMI). We don't think that is terribly relevant, but do believe it will only reinforce the results. We are almost done with that analysis and then will re-submit.
We presented the study at SAEM 2018. Here are the results in modified form. I am not publishing the entire abstract, as it will hopefully be published in the future.
Electrocardiographic Diagnosis of Acute Coronary Occlusion in Ventricular Paced Rhythm Using the Smith Modified Sgarbossa Criteria.
Results: There were 59 OMI subjects and 102 controls (mean age 73 years; male 103 [64%]). The sensitivity and specificity of the MSC versus OSC for OMI were 81% (95% CI 69-90) versus 56% (95% CI 42-69; P<.001) and 96% (95% CI 90-99) versus 97% (95% CI 92-99). Adding concordant ST-depression in V4-V6 to the MSC yielded 86% (95% CI 75-94) sensitivity. For the excessive discordance component, the ratio identified 17 OMI patients vs. 2 for absolute ST Elevation of 5mm.
Conclusions: For the diagnosis of OMI in the presence VPR, the MSC were more sensitive than the OSC; specificity was equivalent.
===================================
MY Comment by KEN GRAUER, MD (10/19/2020):
===================================
Important case by Dr. Walsh that adds to our growing collection of cases in which OMI is evident despite the presence of a pacemaker (Most recently — See our October 5, 2020 post in Dr. Smith’s ECG Blog).
- I limit my comments in today’s case to the two 12-lead tracings obtained by the EMS team. I wanted to begin (as did Dr. Walsh) — by focusing on the 1st ECG shown above (which I have reproduced in Figure-1). Dr. Walsh asked the KEY question about this ECG = “Could cath lab activation be justified from ECG #1?"
Figure-1: The 1st ECG shown above in today’s case. For clarity — I’ve numbered the beats (See text).
MY THOUGHTS ON ECG #1:
As per Dr. Walsh, given the presence of new symptoms (ie, acute dyspnea) in this elderly patient — ECG findings in ECG #1 at the least justify strong consideration of cath lab activation. I’d add the following thoughts to the points highlighted in Dr. Walsh’s excellent discussion:
- POINT #1: As emphasized in “Pearl #1” in My Comment to the October 11, 2020 post in Dr. Smith’s ECG Blog — as many as 30% of all patients with acute MI do NOT have chest pain. Among these patients with “Silent MI” — the most common non-chest pain symptom associated with acute MI (especially among elderly patients) — is shortness of breath (which is the reason the elderly patient in today’s case came to the ED).
POINT #2: Although it is often more difficult to identify OMI in the presence of cardiac pacing — it is not always impossible to do so “just because the patient has a pacemaker”. And sometimes, acute OMI may be obvious despite the presence of a pacemaker. (SEE the links below — among other examples on Dr. Smith’s ECG Blog).
- KEY: In many more cases than is commonly appreciated — modified Smith-Sgarbossa criteria provide an objective means for identifying acute MI despite the presence of cardiac pacing. As per Dr. Walsh — these modified Smith-Sgarbossa criteria are strongly suggestive of OMI in ECG #1.
Among many Other Examples of Acute OMI despite Cardiac Pacing:
- Our October 5, 2020 post.
- Our August 20, 2019 post.
- Our April 25, 2019 post.
- Our October 3, 2018 post.
POINT #3: In addition to modified Smith-Sgarbossa criteria — ECG #1 also shows suggestive Qualitative Criteria for acute OMI. By “qualitative” criteria, I mean that one focuses on ST-T wave shape — and the presence of ST-T wave deviations that simply should not be there in association with a given conduction defect (like LBBB) or with a given paced QRS morphology.
- Becoming comfortable with assessment of qualitative ST-T wave changes in shape provides another way to strongly suspect acute OMI — even when millimeter-determined criteria are not necessarily satisfied.
CAVEAT: The most challenging aspect of assessing ST-T wave morphology in ECG #1 — is the presence of baseline wander with artifact. Specifically — ST-T wave morphology varies significantly from one beat to-the-next in many leads. This makes it difficult to know which one(s) of the 2, 3 or 4 QRST complexes that we see in each of the 12 leads is the one(s) that we should be assessing for potential acute ischemia.
- POINT #4: There is no perfect “rule” for addressing the above caveat. As a result, I favor a “Gestalt” ( = overall) approach — in which one “steps back” and mentally averages ST-T wave appearance for all complexes in each of the leads in a given lead area — all done in context to the overall findings on the 12-lead, with special attention to those leads expected to show reciprocal changes.
- MY DISCLAIMER: Concrete measurable criteria for this “Gestalt Approach” do not exist. Instead, this is that indescribable “sense” that the experienced clinician gets within moments of seeing a patient as to what the diagnosis is likely to be.
Returning to MY Thoughts on ECG #1:
Knowing that today’s case came from an elderly patient with new dyspnea but no chest pain — the following were my thoughts on seeing the paced tracing shown in Figure-1.
- The rhythm in ECG #1 appears to be 100% paced. Regular pacing spikes are seen in many (not all) leads — with 100% capture showing a wide QRS at a regular rate of ~100/minute.
- Looking first at the 6 limb leads in ECG #1 — Assessment of ST-T wave appearance in lead I is not helpful. There is just too much beat-to-beat variation in ST-T wave morphology.
- There is also much beat-to-beat variation in ST-T wave morphology for each of the 4 QRST complexes in eachof the inferior leads. That said — Don’t YOU get a “sense” of disproportionate (ie, more-than-there-should-be) J-point ST elevation in each of these 3 inferior leads (given the modest depth of S waves in these inferior leads)?
- Admittedly — the ST-T wave shapes of beat #1 in lead III — and of beats #5 and 7 in lead aVF do not look abnormal for paced beats. However, even though ST-T wave appearance of other complexes in these inferior leads all differ from one another — I thought they looked suspicious.
- IF the J-point ST depression in beat #6 of lead aVL was real — this would indicate “tell-tale” reciprocal ST depression. The other 3 beats in lead aVL also suggest inappropriate J-point depression — although modest R wave amplitude makes this more difficult to assess.
- I found assessment of chest leads even more challenging. We get at least a glimpse of 5 elevated ST segments in lead V3 (of beats #9-13) — and each of the 5 looks suspicious.
- Each of the 3 ST segments in lead V4 look abnormal — with the amount of J-point ST elevation in beats #14 and 16 seemingly disproportionate to the modest S wave depth in this lead.
- Regarding the other chest leads — lead V1 is of no help — the ST segments of beats #10 and 11 in lead V2 look suspiciously coved, though not overly elevated — and ST segment shape in leads V5 and V6 is clearly abnormal for beat #15, but unimpressive for beats #14 and 16.
- BOTTOM Line: I would not be certain from ECG #1 that this elderly patient with new dyspnea (but no chest pain) was having an acute OMI — but I definitely would be suspicious. I’d want to repeat the ECG (hopefully with less beat-to-beat variation).
As per Dr. Walsh — it turned out the EMS team obtained multiple tracings on this patient. The one they obtained ~10 minutes before ECG #1 was absolutely diagnostic (Figure-2).
Figure-2: Comparison of ECG #1 in today’s case — with a non-paced tracing obtained in the field ~10 minutes earlier (See text).
POINT #5: Serial tracings are often diagnostic. This was especially true in today’s case — in which severe dyspnea resulted in beat-to-beat artifactual variation in ST-T wave morphology.
- Ten minutes earlier — the patient had a spontaneous (non-paced) rhythm. Although hard to appreciate P waves due to the baseline artifact in ECG #2 — subsequent ED tracings showed an underlying sinus mechanism.
- Although there is diffuse low voltage in ECG #2 — each of the inferior leads show subtle-but-real ST elevation. Lead aVL suggests even more subtle reciprocal ST depression.
- Obvious hyperacute ST elevation is seen in leads V4 and V5 of ECG #2. Looking closely — ST elevation appears to begin in leads V2 and V3 — and extend through to lead V6.
- ECG #2 is diagnostic of acute infero-antero-lateral OMI from acute LAD occlusion with “wraparound”.
FINAL Point: Take ANOTHER LOOK at both tracings in Figure-2.
- It’s insightful to compare lead-to-lead paced ST-T wave appearance (in ECG #1) — with what ST-T waves look like in these same leads when there is no ventricular pacing (in ECG #2). My hope is that doing so will help to better appreciate what qualitative ST-T wave findings to look for the next time you encounter a paced tracing in a patient with new symptoms.
Our THANKS to Dr. Brooks Walsh for presenting this highly insightful case!












thank you Dr. Walsh! and you as well, Ken. hopefully no one believes any more that pacers preclude ones ability to discern OMI. sometimes it feels like we are still attempting to come out of the "Dark Ages" of medicine, in that so much has transpired in just the last 50 years.
ReplyDeletei can imagine 100 years from now (if the planet has not been destroyed by us) docs looking back to 2020 and saying "Jeez. the steve smith modified Sgarbossa criteria for pacers and LBBB were not even universally practiced! and they were still using fossil fuels!"
thank you so much to you both.
tom
Thank you Tom! — :)
DeleteP.S. It sometimes takes time for change ...