Written by Jesse McLaren, with comments from Smith
An 85 year old with a history of CAD presented with 3 hours
of chest pain that feels like heartburn but that radiates to the left arm.
Below is the ECG. What do you think?

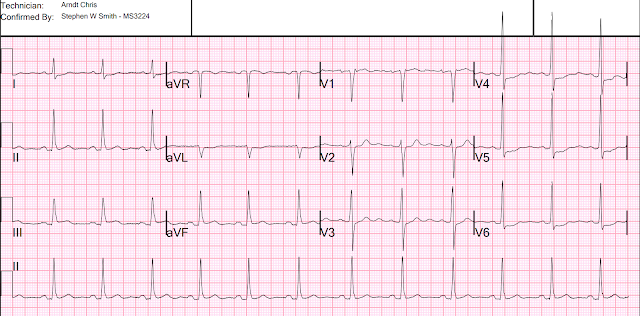
There’s sinus bradycardia, first degree AV block, normal
axis, delayed R wave progression, and normal voltages. There’s minimal concave
ST elevation in III which does not meet STEMI criteria, so this ECG is "STEMI negative". But there are multiple other abnormalities that when combined are diagnostic of OMI and predictive of RCA occlusion:
- sinus bradycardia, which is common in RCA occlusion
- inferior hyperacute T waves (broad based, symmetric, tall relative to the QRS)
- reciprocal ST depression and T wave inversion in aVL (and I), which is highly specific for inferior OMI
- primary anterior ST depression, which is posterior OMI until proven otherwise
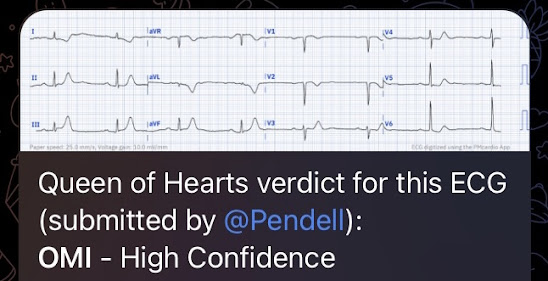
Here's the interpretation of the PMcardio AI trained in identifying OMI:
Below is the old ECG, showing the first degree AV block,
delayed R wave progression and some of the precordial ST depression is old especially in the lateral leads. But
the bradycardia and the infero-posterior OMI is definitely new:
 Smith: this also has many abnormalities suggestive of ischemia: many leads have ischemic appearing ST depression
Smith: this also has many abnormalities suggestive of ischemia: many leads have ischemic appearing ST depression
The emergency provider followed the sequential steps of the current
paradigm:
1.
Use STEMI criteria to identify acute coronary
occlusion: the ECG was STEMI negative
2.
Use troponin to rule out non-STEMI: two high
sensitivity troponin I performed two hours apart were 4 and 16 ng/L, both in
the normal range (upper limit of normal 16 in females and 26 in males). The assay was Abbott Alinity, which is very similar to Abbott Architect high sensitivity troponin I. See analysis below.
3.
Arrange follow up for chest pain patients who
are “STEMI negative” with “normal troponin”: the patient was referred to
outpatient cardiology
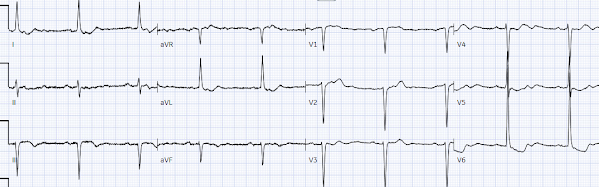
But 6 hours later the patient returned with recurrent chest pain:
Again diagnostic of infero-posterior OMI, though this time
it does STEMI criteria, albeit barely. The cath lab was activated.
A repeat ECG was done on way to cath lab:
"STEMI negative" again. Hyperacute T waves are deflating, suggesting reperfusion but
there is still reciprocal change in I/aVL and ST depression in V2, and the bradycardia is
worse. On angiogram there was a 90% RCA occlusion. Troponin rose from 600 to
17,000 ng/L.
Discharge ECG showed resolution of bradycardia, inferior
reperfusion T wave inversion, and baseline precordial ST depression.
Take home
1.
As the new ACC consensus states (citing the work of Smith/Meyers), "The application of STEMI ECG criteria on a standard 12-lead ECG alone
will miss a significant minority of patients who have acute coronary
occlusion.
Therefore, the ECG should be closely examined for subtle changes that
may represent initial ECG signs of vessel occlusion, such as hyperacute T
waves...or ST-segment elevation <1 mm, particularly
when combined with reciprocal ST-segment depression, as this may
represent abnormal coronary blood flow and/or vessel occlusion."
2.
Using troponin for acute coronary occlusion is
like relying on a rear-view mirror to navigate a car pile-up: it shows wreckage
behind you that has already happened, but can’t see the road ahead and can give false reassurance when there's a head-on collision happening in real time. It’s common for acute coronary
occlusion to present with troponin in the normal range, and the initial rise
can’t predict the final damage. Even if the troponin on the first visit had been higher there still would have been delayed reperfusion because it would have been diagnosed as "non-STEMI"
3.
Using risk stratification tools like HEARTS or
EDACS may have avoided the initial discharge, but shouldn’t be used if the ECG
is already diagnostic of OMI. (See this other post: Chest
pain, a ‘normal ECG’ a ‘normal trop’, and low HEART and EDACS score: discharge
home? Stress test? Many errors here.) There’s also a hazard of relying on
troponins that are in the normal range but above the level of detection. As
this study from Dr. Smith concluded: “measurable hs-cTnI
concentrations less than or equal to sex-specific URLs have important
prognostic implications. Our findings underscore the importance of recognizing
cTn as a continuous variable, with the higher the cTn, the higher the
probability of MACE. We caution against the clinical use of the terms normal
or negative among such patients.” (Clinical
features and outcomes of emergency department patients with high-sensitivity
cardiac Troponin I concentrations within sex-specific reference intervals.)
Smith comments on troponin:
I've done a lot of research on high sensitivity troponin, with colleagues including Fred Apple and Yader Sandoval. We have published over 30 articles, most on high sensitivity troponin, mostly on Abbott Architect high sensitivity troponin I. We have found that, to rule out myocardial infarction (and we mostly only studied Non-OMI), the 2-3 hour delta should be less than 3 ng/L. This conforms with lots of other research done by the HIGH-STEACS group in Scotland and others. In this case, the delta was 12 ng/L.
A delta of 12 ng/L is highly likely to indicate acute MI, even if the value is below the 99th percentile. How is that possible? Because the 3rd or 4th troponin is highly likely to be ABOVE the 99th percentile if the 3 hour value has risen from 4 to 16 ng/L.
See this graphic from one of our papers:
The PPV is particularly low relative to the specificity because this was a very low risk population. In a high risk situation, the PPV would be very high.
Notice that these deltas are REGARDLESS of the initial value. But if the initial value is very low, as in this case, a delta of 12 early in the course of chest pain is even more significant.
Conclusion:
This patient should NEVER have been ruled out by troponin.
And the ECG findings, which are diagnostic of OMI, were also missed.
Thus, this is the protocol Fred Apple and I developed for Hennepin for the Abbott Architect:
==================================
My Comment by KEN GRAUER, MD (3/24/2023):
==================================
I like this case by Dr. McLaren — because it allows us to highlight a very important fault of the outdated STEMI paradigm that is all-too-often forgotten — namely, Being sure to obtain and clinically correlate at least 2 serial ECGs before you send the patient home! (with "clinical correlation" meaning lead-by-lead comparison of these serial ECGs — keeping in mind the presence and relative severity of CP at the time each ECG was obtained).
- For ease of comparison in Figure-1 — I’ve reproduced the first 3 ECGs that were done in today’s case.
In reviewing events transpired in today's case — Obtaining a 2nd ECG and clinical correlation of symptom severity with each ECG that is recorded before discharging the patient was clearly not done on this patient's 1st visit to the ED (Emergency Department). I say this because:
- Considering that today's patient presented with new CP (Chest Pain) — the initial ECG is already diagnostic of an acute event until proven otherwise.
- As noted by Dr. McLaren, compared to the prior tracing — there are a number of new ST-T wave changes in ECG #1.
- There is no notation of whether CP was still present at the time ECG #1 was obtained (and if so, whether CP was increasing, remaining constant, or decreasing). Without this information — it is impossible to understand if the acute-looking ST-T wave changes in ECG #1 might indicate ongoing acute occlusion vs spontaneous reperfusion vs spontaneous reocclusion.
- In addition to the above missteps — the Troponin Delta (ie, the increase in Troponin from 4-to-16 ng/L) that was interpreted as “negative” — is not a "normal" result (as discussed in detail by Dr. Smith). Therefore, even without the acute ECG changes seen in this case — full evaluation of this patient would be needed.
Challenging Aspects of Today's CASE:
Perhaps the most challenging aspect of today's case — is knowing HOW to interpret the initial ECG in light of obvious ECG abnormalities in the prior tracing. Addressing this issue raises the question of how to optimally compare serial tracings.
Regarding Comparison of Serial ECGs:
- I favor picking one of the 2 tracings that you are comparing — and systematically interpreting that tracing in its entirety before you look at the 2nd tracing.
- When comparing a current tracing with a prior ECG — we ideally should know the circumstances under which the prior tracing was done (ie, Was the patient stable and without symptoms? — or — Was the prior tracing obtained during chest pain or soon after an infarction?). Unfortunately — We do not know the circumstances under which the prior tracing in today's case was recorded.
- Are ECG parameters in the 2 tracings you are comparing similar? (ie, Is there a change in the frontal plane axis? Is R wave progression similar? Is the heart rate and rhythm in the 2 tracings the same?). Significant change in any of these parameters may result in ST-T wave changes that are not the result of ischemia or infarction.
Comparison of the 3 Tracings in Figure-1:
The first ECG we were shown in today's case is ECG #1:
- As per Dr. McLaren — there is marked sinus bradycardia and arrhythmia (ie, heart rate in the 40s) — with 1st-degree AV block (PR interval ~0.23 second).
- Regarding other intervals — the QRS is narrow — and the QTc is probably normal given the slow rate. The frontal plane axis is normal (about +70 degrees). There is no chamber enlargement.
Regarding Q-R-S-T Changes: There are artifactual undulations in the baseline of ECG #1 — but this does not prevent interpretation of this tracing.
- There are no significant Q waves (ie, The QS in lead V1 is not abnormal per se). A tiny-but-present initial r wave is seen in lead V2 — with this R wave progressively increasing across the precordium. Transition (where the R wave becomes taller than the S wave is deep) — is slightly delayed (to between leads V3-to-V5).
- ST segments are straightened in multiple leads. In the inferior leads, this is associated with slight J-point ST elevation and clearly hyperacute T waves (that are disproportionately tall, "fat" at their peak — and wider than expected at their base).
- Reciprocal changes (ie, a mirror-image opposite ST-T wave picture) — are seen in lead aVL, and to a lesser extent in lead I. Considering how tiny QRS amplitude is in these high-lateral leads — these have to be considered acute changes until proven otherwise!
- In the Chest Leads — ST-T wave changes are equally concerning. There is ST segment coving with T wave inversion in leads V1,V2. We see a distinct straightening with downsloping of the ST segment in leads V3-thru-V6. This is followed by terminal T wave positivity in these leads — with T waves in leads V3,V4,V5 being clearly "hypervoluminous" ("fatter"-at-their-peak and wider-at-their-base than they should be — as well as disproportionately tall in leads V3,V4 considering R wave amplitude in these leads).
- IMPRESSION of ECG #1: As per Dr. McLaren — Especially in view of the marked bradycardia, the above ECG findings are diagnostic of acute infero-postero OMI until proven otherwise! The ST segment coving in leads V1,V2 suggests possible acute RV involvement — with acute occlusion of the RCA as the presumed "culprit" artery. Given the history of new chest pain — prompt cath is clearly indicated on the basis of this initial ECG.
Comparison of ECG #1 with the Prior Tracing:
As alluded to earlier — ECG #2 is not a normal tracing. Instead — there is ST segment straightening in multiple leads, sometimes with slight ST depression. T waves look disproportionately large in a number of leads (potentially hyperacute IF the patient was having new chest pain at this time). There is ST segment coving with shallow T wave inversion in lead aVL.
- Several differences in ECG parameters make comparison of ECG #1 with ECG #2 challenging. These include: i) The much faster heart rate in the prior tracing; and, ii) Little change in the frontal plane axis — but clearly increased QRS amplitude in the prior tracing.
Looking first at the Limb Leads:
- Although straightening of ST segments is not a new finding in ECG #1 — there should be no doubt that the subtle ST elevation in leads III and aVF is real — since if anything, there was slight ST depression in these leads on the prior tracing. Similarly, the hyperacute T wave appearance in these inferior leads is markedly increased in ECG #1.
- Reciprocal ST-T wave depression with T wave inversion is similarly markedly accentuated in leads I and aVL of ECG #1.
In the Chest Leads:
- Although ST segment straightening with prominent T waves was present in the prior tracing — lead-by-lead comparison suggests that the T waves in leads V3-thru-V6 in ECG #1 are relatively taller (considering QRS amplitude in each respective lead) — and definitely "fatter"-at-their-peak and wider-at-their-base (ie, more hyperacute) than they were in the prior tracing.
- IMPRESSION: In this 85-year old patient with new chest pain — comparison of the prior tracing with ECG #1 should remove all doubt about the acuity of ECG changes on this initial tracing. Prompt cath is clearly indicated — especially in view of the worrisome bradycardia in ECG #1. The patient should not have been sent home.
The Repeat ECG:
As per Dr. McLaren — the patient was unfortunately discharged from the ED — but returned 6 hours later with a recurrence of chest pain. Millimeter-based STEMI criteria are finally attained.
- Comparison of ECG #3 with the initial ECG done 6 hours earlier — and with the "baseline" (prior) tracing, provides insight into the sequence of ECG changes correlated to patient symptoms.
- There is now definite ST elevation in all 3 inferior leads in ECG #3 — in association with T-QRS-D (Terminal-QRS-Distortion — as the S wave in leads III and aVF has been lifted from the baseline) + an even greater increase in relative size of the hyperacute inferior T waves (The T waves in leads III and aVF now tower over the R waves in these leads — whereas they were approximately the same height as the R waves in ECG #1).
- Reciprocal ST-T wave depression/T wave inversion in high-lateral leads I and aVL has increased a comparable amount to the inferior lead ST elevation.
- In contrast — ST-T wave changes look less prominent in ECG #3 than they were on the initial tracing. The evolution of sequential ECG changes during an acute ongoing event is not always homogeneous.
-USE.png) |
| Figure-1: Comparison between the initial ECG in today's case — with a prior tracing — and with the repeat ECG (done 6 hours after ECG #1). |
-USE.png)








-USE.png)
-USE.png)
-USe.png)
-USe.png)
-USE.png)


-USE.png)








-USE.png)