Written by Jesse McLaren, with comments by Smith and Grauer
Four patients presented with
cardiorespiratory symptoms, with inferior ST elevation and reciprocal change on
their ECG. Which patient had occlusion MI?
Note: according to the STEMI paradigm these ECGs are easy, but in reality they are difficult.
First let’s start with each ECG
without clinical context. What do you
think of each ECG?
ECG 1:
ECG 2:
ECG 3:ECG 4:
Now let’s introduce some clinical context. How would this
change management?
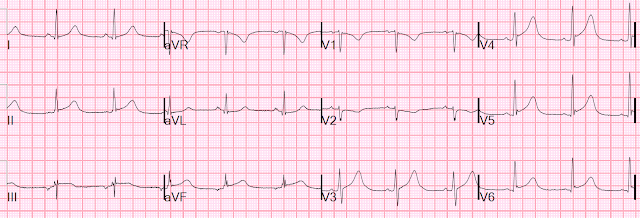
Patient 1: 30 year old previously healthy, presenting with syncope, now
asymptomatic with normal vitals
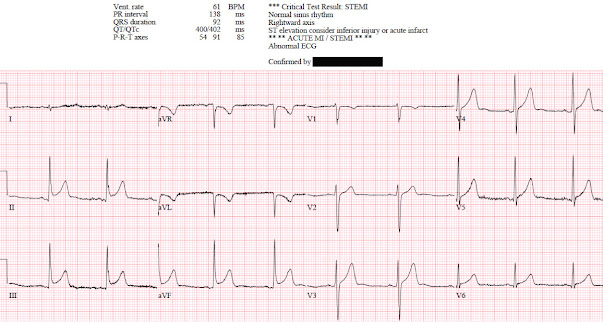
ECG: normal sinus rhythm, normal conduction, vertical axis,
normal R wave progression, no hypertrophy. There’s inferior STE which meets STEMI criteria, but this is
in the context of tall R waves (18mm) and relatively small T waves, and the
STD/TWI in aVL is concordant to the negative QRS. I sent the ECG to Dr. Smith
without any clinical context and he replied: "not OMI" because it looks so much like normal variant.
Because the patient was asymptomatic the emergency physician
didn’t activate the cath lab but got a stat cardiology consult. The patient had
serial ECGs that were unchanged, serial high sensitivity troponin I were
undetectable, an echo was normal, and the patient was discharged with a diagnosis
of vasovagal syncope and a copy of their ECG for future ED visits. So this ECG
false positive STEMI, which could be recognized by expert interpretation.
Smith: The fact that the ECG did not evolve is further proof that this was the baseline ECG.
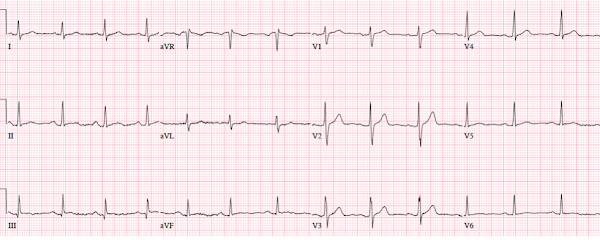
Patient 2: 55 year old with 5 hours of chest pain
radiating to the shoulder, with nausea and shortness of breath 
ECG: sinus bradycardia, normal conduction, normal axis,
normal R wave progression, no hypertrophy. There’s inferior STE in the context
of large R waves and relatively small T waves, and the STD in aVL is concordant
to the QRS complex (i.e. nearly identical to the first case). I sent the ECG to Dr. Smith without any clinical context
and he replied: “not OMI”.
But as he emphasizes, OMI is a clinical diagnosis that does
not depend on the ECG. The patient presented with classic symptoms of acute
coronary occlusion, which may or may not register on the ECG. Because of the
symptoms and ECG the cath lab was activated: 95% distal RCA occlusion, with an
initial troponin I of 200 ng/L (normal <16 in females and <26 in males)
and peak of 13,000. On discharge ECG there is still mild inferior STE but now
reperfusion T wave inversion.
The discharge diagnosis was “STEMI” but the ECG could have
been false positive STEMI. On the other hand, OMI is a clinical diagnosis, and while the ECG
was not diagnostic the patient had ischemic chest pain and OMI was confirmed
based on angiography, peak troponin and reperfusion T wave inversion.
Smith: The fact that the ECG evolved shows that, in this case, the initial ECG which appeared to be normal variant really was due to OMI.
Patient 3: 40 year old with two days of intermittent chest
pain which was both exertional and pleuritic

ECG: normal sinus rhythm, normal conduction, normal axis,
normal R wave progression, no hypertrophy. There’s inferolateral STE in the
context of normal voltages (5-8mm tall), inferior T waves are pointy but large relative to
the QRS (as big as the entire QRS in III), and the STD/TWI in aVL is discordant to its QRS. I sent this to Dr.
Smith without any clinical information and he replied: “OMI”.
The patient had the cath lab activated but had normal coronaries. First
Troponin I was <2 and peak was 8, echo showed subtle apical lateral
hypokinesis, CRP was elevated, and patient was discharged with a diagnosis of
regional pericarditis. There are no prior or follow up ECGs available. This was
false positive STEMI with an ECG mimicking OMI.
Smith: The normal coronaries and negative troponins prove that this was indeed "Not OMI." In this case, there would be evolution, but the evolution would be typical of pericarditis (if the diagnosis of pericarditis was accurate!! Many cases pericarditis are really normal variant, but this ECG does not mimic normal variant, so I am quite certain that this really was pericarditis.)
Patient 4: 35 year old with one hour of chest pain radiating to the left arm, with shortness of breath, nausea and diaphoresis

ECG: normal sinus rhythm, normal conduction, normal axis,
normal R wave progression, no hypertrophy. There’s inferior STE in the context
of normal voltages, with straightening of ST segments and STD in aVL which is
discordant to its QRS. I sent this to Dr. Smith without any information and he
replied, “OMI.”
This was missed by the treating physician, but the chest pain
resolved with aspirin. First troponin I was 7ng/L and repeat was 300. Repeat ECG:
Resolution of STE and reciprocal change, indicating
reperfusion and correlating with resolution of pain.
The patient was referred to cardiology as NSTEMI but had another brief episode of chest pain. As the cardiologist noted, "ECG demonstrated subtle inferior ST segment elevation which normalized and then recurred with further chest discomfort. Code STEMI was called." This repeat ECG is not available in the chart, but sounds similar to the presenting ECG, and it was excellent care that the code "STEMI" was activated despite no evidence of STEMI criteria. The pain resolved again before cath, which found a 80% RCA occlusion. Troponin was 2,000
before cath but no subsequent troponin were done. Discharge ECG showed new Q
wave and reperfusion TWI in III:
Because the patient had the cath lab activated and received
a stent, the discharge diagnosis was “STEMI” even though none of their ECGs met STEMI criteria. This was STEMI(-)OMI.
Smith: in this case, the angiogram and the troponin confirm acute MI (OMI or NOMI), but the evidence that the ECG findings are indicative of OMI is the evolution. The peak troponin did not meet our research definition of OMI (Peak high sensitivity troponin I > 10,000 ng/L, but this is only because there was such rapid reperfusion, with resolution of symptoms.
Inferior STE and
reciprocal change
As Dr. Meyers has explained in a previous
post on inferior STE and reciprocal change in aVL:
“Any
time there is focal STE in the inferior leads for any reason (whether it is due
to acute inferior wall OMI, or whether it is a baseline ECG finding), there
MUST BE reciprocal STD in aVL. The fact that there is STD/TWI in aVL does not
make the STE in the inferior leads specific for active OMI, it just means that
the STE in the inferior leads is indeed focal. If it were diffuse STE on all
walls of the LV, it would be very unlikely to have STD in aVL. Also remember
that Dr. Smith's study on STE in the inferior leads and STD/TWI in aVL was only
comparing patients with inferior OMI against patients with pericarditis. Normal
variants and other reasons for STE in the inferior leads were not in that
comparison.”
This was in reference to this study: primary ST
depression in aVL (ie. Not secondary to LBBB, LVH, or WPW) is 99% sensitive for
inferior OMI, and 100% specific for differentiating inferior STEMI from
pericarditis (but not 100% specific for OMI).
Similarly, these 4 cases all have inferior STE with
reciprocal STD in aVL, but the question is why—including early
repolarization/normal variant, inferior OMI, or myocarditis/focal pericarditis
(diagnosis of exclusion). The STEMI paradigm can’t differentiate between these,
and leads to false positives (case 1) as well as false negatives (case 4), both
of which could be identified by expert interpretation. The other cases show that in the OMI paradigm the
ECG is but one datapoint: you can have clinical OMI without diagnostic ECG (case 2), or OMI ECG mimics that are only ruled out after angiography (case 3).
Take home
1.
The STEMI dichotomy leads to false positives and false negatives and
puts the focus exclusively on the ECG, while the OMI paradigm can
differentiate subtle occlusions from mimics while putting the ECG in the
context of the patient.
2. Primary reciprocal STD in aVL is highly
sensitive for inferior OMI (far better than STEMI criteria) and excludes
pericarditis, but is not specific for OMI
3. Normal variant STE can mimic STEMI but has tall
R waves and relatively small STE and T waves; TWI in aVL can be normal if
concordant to an inverted QRS (eg in vertical axis)
4. Myocarditis and regional pericarditis are
retrospective diagnoses of exclusion after a normal angiogram
===================================
MY Comment by KEN GRAUER, MD (5/23/2022):
===================================
Today's post by Dr. McLaren is a perfect follow-up for the May 11, 2022 post by Dr. Meyers, in which Dr. Meyers asked us to identify which of the 5 tracings he showed were suggestive of acute OMI.
In today's post — Dr. McLaren puts us to a similar challenge, namely to identify which of the 4 tracings he shows with inferior lead ST elevation are suggestive of acute OMI?
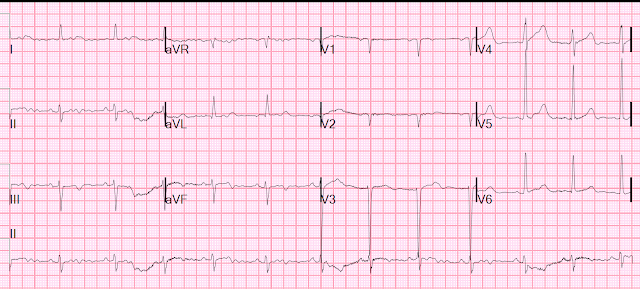
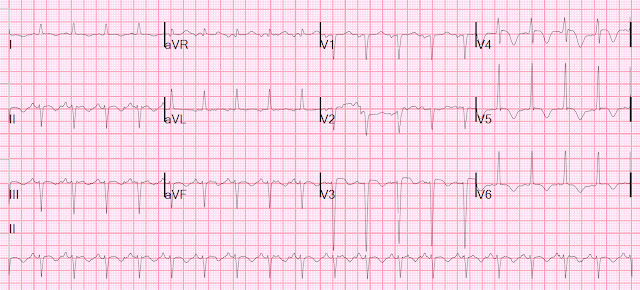
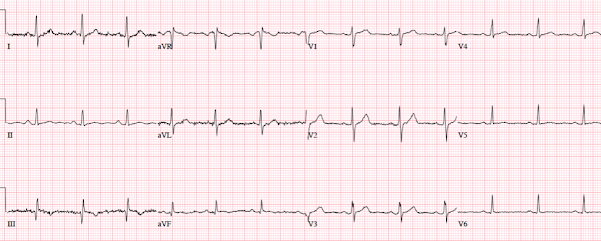
- I picked 2 of the 4 Cases to comment on. For clarity — I've put these 2 tracings together in Figure-1.
- Once again — We were initially shown these tracings without the benefit of any history. Dr. McLaren then filled us in on the clinical setting — and on "the Answers" to this clinical question.
-USE.png) |
| Figure-1: I've reproduced ECG #1 and ECG #2 from today's post. |
Looking First at ECG #1:
My purpose in commenting on ECG #1 is twofold: i) As per Dr. McLaren — this ECG manifests typical features of ERP (Early Repolarization Pattern); and, ii) Once you feel comfortable identifying this tracing as suggestive of ERP (and not suggestive of acute OMI) — the important Question then becomes, "What do you do for a young patient who presents to an ED for syncope with an ECG that clearly shows Early Repolarization?"
- ECG findings of a repolarization variant that ECG #1 shows include upward-sloping ("smiley"-configuration) ST segment elevation in multiple leads (especially in the inferior leads — but also to a lesser extent in leads V3-thru-V6).
- No J-point notching — but slurring of the downslope of the R wave in 2 contiguous leads (ie, in leads II and III — as shown by the PURPLE arrows that mark the point of onset of this slurring).
- Against acute OMI are: i) Excellent R wave progression (with an R wave amplitude over 10 mm by lead V3); ii) A relatively short-normal QTc interval; and, iii) Absence of reciprocal ST depression, in the presence of similar-looking peaked T waves of normal amplitude in no less than 8 leads (II,III,aVF; and V2-thru-V6) — whereas the ECG of an acute OMI is much more likely to localize.
- To emphasize — the inverted T wave in lead aVL of ECG #1 "looks" benign — since the T wave vector often follows close behind the QRS vector (ie, the negative T wave in lead aVL "follows" the negative QRS in this lead) — AND — the shape of this inverted T wave peak looks comparable to the shape of the T wave peak in those leads in which the T wave is positive.
- BOTTOM Line: The history in this young adult was syncope and not chest pain — so there is a "lower prevalence likelihood" by history for acute OMI. In view of this lower prevalence likelihood for acute OMI — the above description is my best attempt at explaining why my brain immediately recognized the pattern in ECG #1 as a non-OMI repolarization variant.
- QUESTION: But what about the history of syncope that brought this patient to the ED? (See below).
What About ECG #2?
I did not know the history when I first saw ECG #2. After looking at this tracing — I was not at all comfortable ruling out acute OMI from this ECG alone.
- The small and narrow q waves in inferolateral leads looked like "normal septal q waves" (which can be seen as a normal finding not only in lateral leads — but also in inferior leads when the frontal plane axis is vertical, as it is in ECG #2).
- I thought the ST segment elevation in each of the inferior leads of ECG #2 looked similar to the inferior lead ST elevation in ECG #1.
- That said — I thought the flat ST segment, with a hint of ST depression in lead aVL was different than the negatively peaked T wave inversion in lead aVL of ECG #1. I thought this to be a nonspecific sign — but it did make me question IF the shape of the ST segment in lead aVL of ECG #2 might reflect a less typical, but nevertheless reciprocal change.
- The KEY finding that I thought was definitely abnormal in ECG #2 — was the straightened ST segment in lead V2 (RED line in Figure-1). Normally — there should be a gradual upsloping of the ST segment, usually with at least slight ST elevation in lead V2.
- I was less sure of what to make of the 0.5-to-1.0 mm ST elevation in leads V3-thru-V6.
- And then there was the history in this 55-year old patient — which was 5 hours of radiating chest pain associated with nausea and dyspnea.
- BOTTOM Line regarding ECG #2: I was concerned about the appearance of ECG #2 before I knew the history. Once the history was revealed — I interpreted this case as one in which acute OMI needed to be ruled out (rather than the other way around) — because: i) There was 1-1.5 mm of ST elevation in each of the inferior leads — with a straightened and slightly depressed ST segment in lead aVL that I thought could reflect a reciprocal change; ii) Lead V2 showed definite abnormal ST flattening (instead of an upsloping ST segment with slight elevation) — which I interpreted as possible (if not probable) indication of posterior wall involvement; iii) There was slight-but-real ST elevation in leads V3-thru-V6 of uncertain etiology; and, iv) This 55-year old patient presented with a classic history of acute cardiac chest pain.
What To Do with a Young Adult who presents with Syncope and ERP?
Once we can become comfortable that ECG #1 is not suggestive of acute OMI — we need to address the question of, "What do you do for a young adult patient who presents to an ED for syncope with an ECG that clearly shows Early Repolarization?"
- The problem is the very small-but-present percentage of lethal cardiac arrhythmic events in otherwise healthy young adult individuals who have repolarization variants. As a result — I no longer use the term "BER" (Benign Early Repolarization) when I encounter the type of ECG finding seen in ECG #1 of today's case (Zakka & Refaat-ACC-2016). Instead, I favor calling this a "repolarization variant" — with awareness that this ECG finding is usually but unfortunately not always 100% "benign".
Distinction is made between:
- ERP (Early Repolarization "Pattern") — in which the ECG findings of early repolarization are seen in the absence of symptomatic arrhythmias.
- ERS (Early Repolarization "Syndrome") — in which early repolarization ECG findings are seen in a patient with a history of PMVT (PolyMorphic VT) or resuscitated idiopathic VT/VFib.
- NOTE: Early repolarization is included among the "J Wave Syndromes" (JWS) — which are a phenotypic spectrum of disorders including individuals with ERS, Brugada Syndrome — and/or — malignant arrhythmias linked to STEMIs or the J-point accentuation (Osborn wave) of hypothermia.
The CHALLENGE:
- In 2008 — Haïssaguerre found an increased prevalence of ER in patients with idiopathic VFib (N Engl J Med 358:2016-2023, 2008). Since then — the challenge has been to try to distinguish between the very common ECG finding of early repolarization vs asymptomatic individuals with ERP who have a significant risk of developing a malignant arrhythmia. The subject is complex — and beyond the scope of the basics that I cover here. Suffice it to say — Much remains to be learned!
I found articles by Di Diego JM & Antzelevitch C (J Wave Syndromes as a Cause of Malignant Cardiac Arrhythmias — Pacing Clin Electrophysiol 41(7): 684-699, 2018) — and — by Ali et al (Early Repolarization Syndrome: A Cause of Sudden Cardiac Death — World J Cardiology 7:466-475, 2015) to be insightful. That said — the BEST article I found regarding definition of the terminology associated with ER (Early Repolarization) — is in the 2015 Consensus Paper written by Macfarlane et al, and including contributions from the biggest names in this field (J Am Coll Cardiol 66:470-477, 2015). Among the points brought out by these articles, and other literature I've reviewed: - The prevalence of ER in the general population has been cited to range between 2-to-31% — with this percentage depending on age, sex, race, differing terminology, and especially the amount of J-point elevation accepted as "qualifying" for the definition. But regardless of the criteria for inclusion that are chosen — Point #1 is that the ECG finding of Early Repolarization is common! — Point #2 (as emphasized by Macfarlane et al in their 2015 Consensus Paper) — is that the definition of "ER" had to be revised and agreed upon by researchers and clinicians in the field.
- Early Repolarization (by the newer definition) — is said to be present on ECG if each of the following Criteria are met: i) There must be an end-QRS notch (J wave) or "slur" on the downslope of a prominent R wave — and this notch or "slur" must lie entirely above the baseline; ii) The peak of this notch (Jp) must be ≥0.1 mV ( = 1mm) elevated above the baseline in ≥2 contiguous ECG leads (excluding leads V1-V3). IF there is a "slur" (instead of a notch) — the onset of the slur must be ≥0.1 mV ( = 1mm) elevated above the baseline; and, iii) These ECG findings should be associated with prominent T waves (in ≥2 contiguous ECG leads) — and — a QRS duration (measured in leads that do not show a slur or a notch) that is less than 0.12 second in duration.
- ST segment elevation without notching or slurring (as specified by the above criteria) — should not be considered "ER" by the Consensus Paper authors. Instead — they favor the term nonspecific ST elevation.
- MY Thought: If such nonspecific ST elevation not qualifying as "ER" is found in an asymptomatic subject with a low-risk profile — perhaps the designation repolarization variant may be equally suitable.
- Di Diego & Antzelevitch emphasize: Incidental discovery of a J wave on a routine ECG recording should not be interpreted as a marker of "high risk" for sudden death (because the overall probability of malignant arrhythmia is very low in the general population = approximately 1/10,000). That said — there are certain characteristics of concern that increase risk.
Figure-2 is adapted from figures in the Di Diego & Antzelevitch article — in my attempt to illustrate ECG findings described with ER, as well as to summarize what goes into the complex process of risk assessment. Some pertinent points include:
- ER (Early Repolarization) findings limited to the lateral ECG leads are extremely common — but are generally associated with low risk of malignant arrhythmias.
- ER associated with a rapidly ascending (upsloping) ST segments are the most common form — and are also generally associated with low risk of malignant arrhythmias.
- A typical low risk patient profile for ER is asymptomatic status (ER being incidentally discovered, when for whatever reason an ECG is done) — younger adult age — negative family history — voltage for LVH on ECG — and, lower heart rate with lower blood pressure (suggesting a healthy, physically active individual).
- Risk is increased when ER findings are seen in the inferior leads (either isolated to inferior leads — or seen in both inferior and lateral leads).
- Risk of malignant arrhythmias is especially increased when ER findings are associated with a horizontal or descending ST segment (See Figure-2).
- It is uncommon to see global J waves (ie, present in inferior, lateral and anterior leads) — but when this occurs, the risk of malignant arrhythmias is extremely high.
As best I can tell from the literature I reviewed — risk of malignant arrhythmias is not reliably predicted by distinction between a slurred vs notched ER pattern (with or without up to 2mm of J-point elevation).
- BUT — Future risk of a malignant arrhythmia is clearly influenced by a patient's personal history (ie, of any worrisome cardiac arrhythmia or potential cardiac-related syncope/presyncope that the patient may have experienced) — and — by family history (ie, of sudden death or significant arrhythmic event).
- A higher risk patient profile would also include being older than "young adult age" — especially if ER findings include horizontal or descending ST segments (See Figure-2) — and — if the ECG also has findings suggestive of coronary disease.
How Does this Relate to the Patient with ECG #1:
All we are told is that this previously healthy 30-year old patient presented with "syncope" — but was asymptomatic at the time ECG #1 was obtained. Workup (including Echo) was negative for OMI — and the patient was discharged from the ED with a diagnosis of "vasovagal syncope" and a copy of their ECG "for future ED visits".
- In my experience — the diagnosis of "vasovagal syncope" is all-too-often a diagnosis of exclusion used when no other acute cause has been found. But without additional information about this case — Can we really be certain there is no potential cardiac-related cause of this patient's syncope? (ie, Symptoms were concerning enough that this resulted in a visit to the ED!).
- Regarding the ER findings in ECG #1 — Risk is at least somewhat increased because of the inferior lead location of the high-takeoff J wave slurring in Figure-1. On the positive side — the ST segment in all infero-lateral leads that show ST elevation is clearly "ascending" (upsloping) — and not horizontal or descending.
- BUT — because this patient presented to an ED for "syncope" — i) It is essential to obtain a thorough personal history (for potential prior events) — and — a thorough family history (for sudden death or malignant arrhythmia) — and — ii) Greater detail should be sought out until a diagnosis of simple "vasovagal syncope" can be made with more certainty.
-USE.png) |
| Figure-2: I've adapted this Figure from 2 figures in the excellent review by Di Diego & Antzelevitch on J Wave Syndromes as a Cause of Malignant Cardiac Arrhythmias (Pacing Clinical Electrophysiology 41(7): 684-699, 2018 ). NOTE — The amplitude of a notch is measured from the peak of the J wave (Jp) or from the onset of the slur (Jp). ST segment slope is assessed from the end of the notch or slur (Jt) — looking to see if the ST segment is ascending, horizontal or descending at a point measured 100 msec. ( = 2.5 little boxes) after the end of the notch or slur (ie, after Jt). |
-USE.png)
-USE.png)
-USE.png)









-USE.png)
-USE.png)
-USE.png)
-USE.png)
-USE.png)





-USE.png)
-USE.png)