A man in his early 30s was walking when he developed central chest pain which was non-radiating, then had a syncopal event with bowel incontinence, and when he woke up he had ongoing chest pain. Notes never having symptoms like this before, pain is so severe its causing SOB.
He called 911. Medics recorded a BP of 79/52 with pulse of 47.
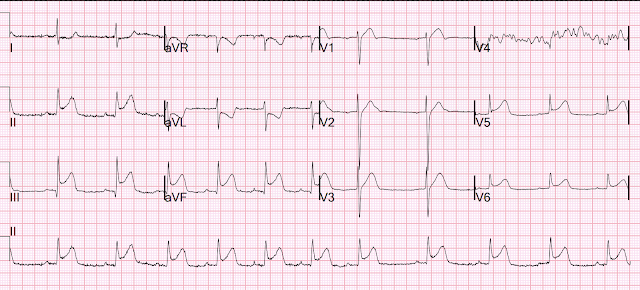
They recorded this ECG:
There is also STE in V1 which is diagnostic of right ventricular OMI in this situation, and partly explains the syncope and hypotension (along with the bradycardia).
A second ECG was recorded by medics:
So this is an obvious proximal RCA OMI, with inferior and right ventricular OMI.
The cath lab was activated by the medics.
He arrived in the ED and had this ECG recorded:
The first high sensitivity troponin I returned undetectable (<3 ng/L).
The angiogram was normal
The post angiogram ECG is here:
Here it is annotated in red:
Our extremely smart radiologist, Gopal Punjabi, assures me that this finding can only be due to myocardial infarction, not myocarditis.
The patient was found to have an embolic source. It was more interesting than that but in the interest of privacy I cannot give more details.
So this is "MINOCA". Myocardial Infarction with Non-Obstructive Coronary Arteries.
Etiologies (list not comprehensive):
Coronary Spasm. Provocative testing is very helpful for this
Coronary Thrombus with lysis (one must do optical coherence tomography or at least intravascular ultrasound to find thes non-obstructive plaques that ruptured.
Embolism with lysis. Need to find an embolic source. Emboli frequently do not lyse and need to be intervened upon.
Coronary microvascular dysfunction
Coronary dissection
Read more about MINOCA and its etiologies here:
https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2022.1032436/full
MY Comment, by KEN GRAUER, MD (4/13/2025):
- As I wrote in My Comment in the November 16, 2023 post in Dr. Smith's ECG Blog — "I suspect that the entity known as MINOCA is not fully appreciated by many clinicians." I suspect this statement is still true 2 years later, in 2025.
- An estimated 5-15% of patients (depending on the studied population) who are diagnosed with either STEMI or NSTEMI — turn out to have MINOCA, in which cardiac catheterization fails to reveal any infarct-related artery (ie, no "culprit" vessel with at least 50% stenosis) — and no clear systemic etiology is found to explain the patient's presentation to the hospital (Tamis-Holland et al: AHA Scientific Statement on MINOCA — Circulation 139:e891-e908, 2019 — Broncano et al — RadioGraphics 41:8-31, 2021 — and — Sykes et al — Interventional Card Review 16:e10, 2021).
- As a result of how surprisingly common MINOCA is — We need to know about this entity if we hope to optimally treat the surprisingly large population of patients who have MINOCA.
- Is there evidence that an acute OMI has occurred?
- If so — Was timely cardiac cath "negative" for confirming acute infarction? If so — then the patient has MINOCA.
- The history in today's case strongly suggests an acute cardiac event: The patient had sudden onset of severe and persistent Chest Pain.
- The initial Troponin was negative. BUT — as we often emphasize, the initial Troponin may be negative in a significant percentage of acute MIs! The repeat Troponin requested by Dr. Smith removed all doubt about whether there was acute infarction (ie, as repeat Troponin = 49,000 ng/L).
- Despite the poor resolution of the 2 EMS ECGs — the diagnosis of acute RCA OMI is immediately established by these EMS ECGs until proven otherwise.
- The cardiac cath was "normal".
- The Unmistakeable Conclusion: By the definition I suggested above — today's patient manifests MINOCA.
- Rather than trying to "pretty up" today's EMS ECGs with PMcardio — I decided to preserve the originals, albeit with some lightening of the background "noise".
- I am uncertain about the rhythm in both EMS tracings, other than to say that the rhythm is supraventricular (narrow QRS) — and both slow and at least fairly regular (rate <50/minute). I simply cannot reliably make out atrial activity. That said — awareness that the rhythm in these 2 tracings is fairly regular and supraventricular suffices for identifying the KEY finding (as in the next bullet below).
- Marked ST elevation in each of the inferior leads — in association with reciprocal ST depression in leads aVL and I — defines this tracing as an inferior STEMI.
- Acute RCA occlusion is strongly suggested by the presence of ST elevation in lead III>II, and the marked reciprocal ST depression in aVL. In this context (as per Dr. Smith) — the abnormal ST segment straightening, elevation and terminal T wave inversion in lead V1 in the setting of acute RCA OMI (within the RED rectangle) — defines acute RV involvement (and tells us that it is the proximal RCA that is occluded at the time ECG #1 was recorded).
- Advanced Point: I suspect the ST coving without either elevation or depression in lead V2 — is the result of attenuation of forces (ie, some cancelling out) of the ST elevation that we would have seen in lead V2 from the acute RV MI — with the ST depression that we otherwise would have seen if there was no RV MI, because of associated posterior OMI.
- P.S.: Given clear indication of acute RV MI on EMS ECG #1 — sublingual NTG should be avoided! And, since a hypotensive BP = 79/52 was recorded by the paramedics — Consideration should be given to the potential need for IV fluids. (For more on the ECG diagnosis and consequences of acute RV MI — Check out My Comment in July 19, 2020 post and the August 2, 2024 post).
- The principal difference that I see in EMS ECG #2 — is in the chest leads, where: i) Lead V1 has obviously become more "bulky" (from the acute RV MI); ii) There now appears to be flattening of the ST segment in lead V2, now with terminal T wave positivity (presumably reflecting more activity from the ongoing associated posterior OMI); and, iii) Leads V3-thru-V6 all look slighty (subtly) more hyperacute, suggesting associated lateral MI — perhaps reflecting compromised perfusion of postero-lateral branches from the PDA.
- None of these subtle changes in the chest leads of EMS ECG #2 alter in any way the conclusion we immediately arrived at on seeing EMS ECG #1 (namely, that there is acute RCA occlusion with acute RV involvement in this acute inferior STEMI ).
- The importance of the points cited below is highlighted by today's case, in which: i) Consideration of the differential diagnosis for MINOCA (shown in Figure-2) — facilitated identification of an embolic source as the cause of the acute MI in today's case; ii) As per Dr. Smith — the simple clinical reality that the ECG changes of an acute STEMI do not disappear within 2 hours immediately ruled out acute myocarditis as a cause; and, iii) Despite acute OMI with spontaneous reperfusion remaining the most common "cause" of MINOCA — sequential evaluation ruled this out as the cause in today's case.
- An all-too-common misconception is that the absence of obstructive coronary disease on cardiac catheterization rules out acute coronary occlusion as the cause of the patient's acute event. This is not the case.
- To paraphrase Dr. Smith's comments in the May 19, 2020 post: — Non-obstructive coronary disease does not necessarily imply no plaque rupture with thrombus. This is because non-obstructive plaques can fissure, thrombose, totally (or near totally) occlude, have autolysis (spontaneous lysis of thrombus with reperfusion) — yet have less than 50% obstruction at angiography. These plaques will often not be recognized as "culprits", because no fissuring or ulcertaion is seen. As a result — determination of plaque disruption in such patients can only be diagnosed by use of intracoronary imaging — with either higher-resolution OCT (Optical Coherence Tomography) or IVUS (Intra-Vascular Ultra-Sound).
- Bottom Line: Despite lack of obstructive coronary disease on cardiac catheterization — the most common cause of MINOCA is probably still an acute OMI that spontaneously reperfused, and was no longer evident by the time cath was performed.
- The 3 most common causes of ACS (Acute Coronary Syndrome) without coronary disease are: i) Myocarditis (up to 1/3 of these patients); ii) Takotsubo cardiomyopathy; and, iii) MINOCA.
- There is a trend toward these patients being younger — with a greater relative percentage of women — and fewer traditional cardiac risk factors.
- Longterm prognosis of patients with MINOCA clearly depends on the underlying etiology — but it's important to appreciate that this entity is not benign, with similar mortality as for patients with obstructive coronary disease following their infarction.
- Cardiac MRI — provides an answer to the etiology of patients with MINOCA in more than 2/3 of cases.
- Cardiac MRI successfully identifies ~80% of patients with acute myocarditis by picking up evidence of inflammation — with the distinct advantage of being noninvasive compared to endomyocardial biopsy.
- Use of LGE (Late Gadolinium Enhancement) — is routinely recommended with cardiac MRI to increase diagnostic yield, as a means to identify fibrosis and other abnormalities in cardiac tissues.
- Cardiac MRI (especially with the addition of LGE) provides insight to longterm prognosis of patients with MINOCA.
-USE.png) |
| Figure-2: Classification of Underlying Diagnoses in Patients with MINOCA (Adapted from Table-1 in Sykes et al: Interventional Cardiology Review: 16:e10, 2021). NOTE: As per Sykes et al — The entities listed under "Other Etiology" may be diagnosed following further investigation and should be considered separately (because they are typically associated with myocardial injury but not considered an MI by the 4th universal definition of MI). This is an important indication for cardiac MRI in patients suspected of MINOCA. |






-USE.png)




-USE.png)





-USE.png)