This case is by Jesse McLaren (@ECGCases), with comments by Smith and Grauer
A 50 year-old with CAD and ESRD went to their regular hemodialysis appointment complaining of two days of exertional chest pain. The patient was sent to the ED when high-sensitivity Troponin I returned at 1,526 ng/L (normal <26 in males, <16 in females). They were painfree on arrival, with BP 180/70 and other vitals normal. What do you think?
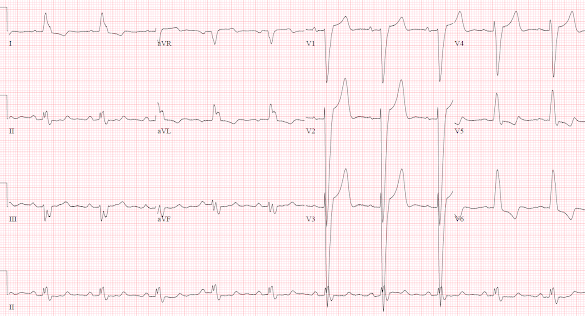
There’s sinus rhythm with LBBB and appropriate discordant ST changes: there’s no concordant ST elevation, no concordant ST depression in V1-3, and no excessive discordance.
Because of the chest pain and positive troponin the patient had a stat cardiology consult in the ED, which noted that the ECG revealed “known LBBB now with >5mm discordant ST elevation V2-3, positive by Sgarbossa criteria”. The cath lab was activated, and a 70% circumflex lesion was stented. The troponin in the ED three hours after the initial troponin was 1509, and continued to fall. The patient had chest pain + ST elevation + elevated troponin + culprit lesion, so was discharged with a diagnosis of “STEMI”. But did they have an Occlusion MI?
Acute coronary occlusion is diagnosed based on clinical presentation (ischemic symptoms), ECG evolution (occlusion and reperfusion), angiographic findings (TIMI 0-2 flow), and significant troponin elevation. But no factor alone is sufficient: patients can have resolved symptoms but ongoing ECG evidence of occlusion, or refractory ischemia with electrocardiographically silent occlusion, or an open artery by the time of the angiogram with a massive troponin from prior occlusion, or a small troponin elevation after rapid reperfusion with ECG signs of occlusion/reperfusion.
Clinical: if the patient presented with refractory ischemia or hemodynamic instability they would have required urgent angiography regardless of ECG findings. But they were pain free and hemodynamically stable.
ECG: they were noted to have a known LBBB. But whether the LBBB is new or old is not relevant, as “ED patients with new or presumed new LBBB are not at increased risk of AMI.”[1]. They were also noted to meet Sgarbossa criteria with >5mm of discordant ST elevation. But the original Sgarbossa criteria applied to any AMI as diagnosed by CK-MB, not occlusion MI diagnosed by angiography and troponin—and even this definition required a score of 3 whereas discordant STE >5mm only gave a score of 2. [2] Unweighted application of the Sgarbossa criteria results in lower specificity, but the Smith-modified Sgarbossa criteria are both sensitive and specific. It defines excessive discordance relative to the QRS (STE/S>25%), and is based on OMI defined by angiography and troponin level.[3] In the validation study, STE/S>25% had a positive likelihood ratio of 99.6, and negative likelihood ration of 0.20; using STE/S>20% still had a positive likelihood ratio of 15, which can be helpful in high pretest likelihood patients, while the negative likelihood ratio was 0.16.[4]
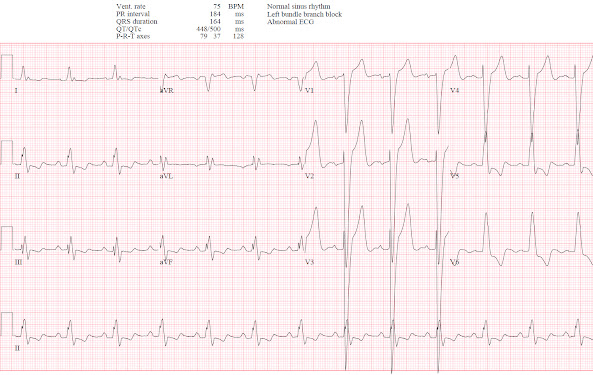
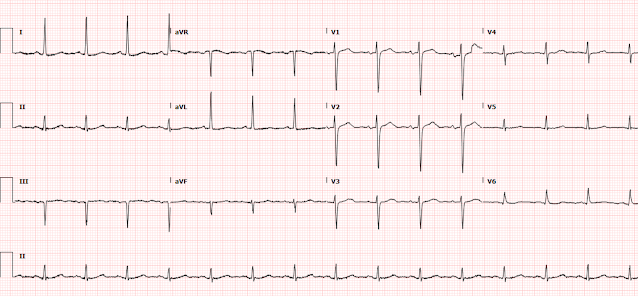
Below are the old, new, and discharge ECGs of the patient above.
Angiogram: there was a hazy 70% circumflex lesion which was stented, along with a 60% LAD stenosis and 40% RCA stenosis. This confirms that the concerns for anterior ST elevation were unwarranted. The patient had the cath lab activated for anterior ST elevation but there was no LAD occlusion. Circumflex occlusions can be electrocardiographically silent, but if they manifest on ECG will produce concordant ST depression (from posterior MI), or concordant ST elevation (from lateral MI), neither of which were present on ECG. A 70% culprit lesion could still be compatible with an OMI that has reperfused by the time of the angiogram, but there was no ECG evolution to suggest this and no significant troponin elevation (e.g. >10,000 ng/L).
Troponin: in OMI the initial troponin can be normal but can soar into the tens of thousands (ng/L for high sensitivity assay). A rapidly reperfused OMI can have a lower peak troponin. But this patient's mildly elevated troponin was already starting to fall by the time they arrived in the ED, which correlates with their resolved symptoms and lack of ECG changes.
In summary, this patient was diagnosed as "STEMI" because of chest pain + ST elevation + troponin + culprit lesion. But the pain had resolved, the ST elevation was proportional, the artery was open and the troponin elevation was mild. This "STEMI" was a NOMI. The patient was taken for urgent cath based on false positive Sgarbossa criteria and elevated troponin level, and diagnosed with “STEMI” based on a culprit lesion that was stented. But they did not have clinical or ECG evidence of an acute coronary occlusion on presentation; and the ECG evolution, angiographic findings and peak troponin confirmed the diagnosis of NOMI.
Contrast with this case, where a patient had clinical and ECG evidence of OMI—with refractory ischemia and positive Modified Sgarbossa Criteria—but did not initially meet traditional Sgarbossa criteria and died after delayed cath lab activation. This shows that the OMI paradigm can optimize cath lab decisions to target those with occluded arteries in need of emergent reperfusion, and differentiate them from those with non-occlusive MI.
Take home:
1. OMI is based on clinical factors, timing of symptoms with ECG findings, ECG evolution, angiographic findings, and troponin levels
2. Patients with LBBB don't need the cath lab for ‘new’ LBBB or discordant STE >5mm if this is proportional to the preceding QRS
3. Patients with LBBB do need the cath lab for refractory ischemia or hemodynamic instability, Smith-modified Sgarbossa (concordant STE, concordant STD in V1-3, or excessively discordant STE defined as STE/S>25% or >20% in high-pretest probability patients
4. Type 1 MIs should be categorized as OMI vs NOMI not STEMI/NSTEMI in order to optimize reperfusion decisions
References
1. Chang et al. Lack of association between left bundle-branch block and acute myocardial infarction in symptomatic ED patients. Am J Emerg Med 2009
2. Sgarbossa et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle branch block. NEJM 1996
3. Smith et al. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med 2012
4. Meyers et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: a retrospective case-control study. Am Heart J 2015
================================
One last comment by Smith about Jesse's case, the Smith Modified Sgarbossa Criteria, and about Ken's comment below:
The diagnosis of OMI, even in LBBB, goes way beyond ST Elevation, and that means that it goes way beyond just measuring the ST/S ratio, or concordance, in LBBB. In Ken's case below, he gives an example of lead aVF being diagnostic due to the hyperacute T-wave, and also a hyperacute T-wave in V6. But in that ECG, the case is POSITIVE by the Smith modified criteria: the ST/S ratio in aVF is 1/4 or 1/3.5, either of which are ≳ 0.25 and definitely greater that 0.20. It only takes one lead to be above the given ratio!
When I try to get into nuance of the ST/S ratio on the criteria, I tell people that our data shows that the highest NORMAL ST/S ratio among the 12 leads is, on average, 0.11. And I say I get worried about any value above 0.15 and that 0.20 is VERY specific for Occlusion, and even more sensitive than 0.25.
We found that the Smith Modified criteria are far better at diagnosing OMI in LBBB than ST Elevation criteria are at diagnosing OMI in normal conduction (which to most who know the history of LBBB should be shocking), and this is because the latter do not take proportionality into account.
See this amazing case:
A Fascinating Demonstration of ST/S Ratio in LBBB and Resolving LAD Ischemia
===================================
MY Comment by KEN GRAUER, MD (3/31/2022):
===================================
Excellent presentation by Dr. Jesse McLaren — which illustrates "the art of electrocardiography" in assessing the patient with chest pain and underlying LBBB. As emphasized by Dr. McLaren — determining whether or not acute OMI has occurred in such a patient consists of much more than simply "the sum of its parts".
- I first learned to appreciate qualitative aspects for assessing the acuity of ST-T wave changes with LBBB in the 1980s from my mentor, Dr. Barney Marriott. Dr. Marriott showed us numerous examples of LBBB tracings with primary ST-T wave changes more than what should be expected with simple LBBB. Although some examples were subtle — others were obvious — yet generally ignored by the cardiology community.
- I remember waiting for years in the hope that some literature would be forthcoming to justify recognition of acute MI in association with LBBB. Finally, a decade later (in 1996) — Sgarbossa et al published their NEJM article with his now well-known and frequently cited critieria (NEJM 334:481-487, 1996).
- As helpful as the original Sgarbossa criteria were for recognition of acute coronary occlusion — these criteria failed to incorporate the concept of proportionality. For this, we are indebted to Dr. Smith for developing Modified Smith-Sgarbossa Criteria — for which, instead of using an absolute measurement of 5 millimeters of ST elevation as the criterion for "abnormal" — a ratio of the amount of J-point ST segment elevation compared to S wave depth was used. The beauty of this addition to the original Sgarbossa criteria — is that it facilitates assessment of LBBB tracings with extremely deep anterior S waves.
- As per Dr. McLaren — the ratios of 6/43 (14%) and 6/53 (11%) respectively, allow instant dismissal of the amount of anterior J-point ST elevation as not at all suggestive of acute coronary occlusion.
- PEARL: As helpful as Smith-Sgarbossa Criteria can be — I like to look for ST-T wave changes that I know are not normal in a patient with LBBB. To illustrate this concept — I've reproduced the initial ECG in the September 17, 2020 post in Dr. Smith's Blog (BOTTOM tracing in Figure-1). The patient complained of new chest pain. The ECG showed sinus rhythm with LBBB.
- I've enclosed within dotted RED rectangles QRST complexes in the 2 leads that most caught my eye. Fully aware that assessment of ST-T wave changes is often extremely challenging with LBBB — there is no way that the J-point notch, with straightened ST segment takeoff and disproportionately "fattened" T wave peak in lead aVF is normal. In view of the tiny QRS amplitude in this lead — the ST-T wave in lead aVF is clearly hyperacute!
- The other QRST complex in ECG #2 that is clearly not "normal" for LBBB — is in lead V6. With typical LBBB — the ST-T wave should be oppositely-directed to the last QRS deflection in lateral leads I and V6. The T wave should not normally be upright in lateral lead V6 when the last QRS deflection is positive (as it is in ECG #2).
- Knowing that there are hyperacute T waves in leads aVF and V6 in this LBBB patient with new chest pain facilitates recognition of other less obvious but clearly abnormal ST-T wave findings in neighboring leads. As per the RED arrows that I've added to ECG #2 — the other 2 inferior leads also show abnormalities (ie, more-than-expected J-point ST elevation in lead III — and a T wave in lead II that is unexpectedly positive and looks hyperacute). Although in isolation, I might not be impressed by the J-point ST depression in high lateral leads I and aVL — in the context of new chest pain and hyperacute inferior T waves — I interpreted this J-point depression in leads I and aVL as consistent with reciprocal ST depression.
- Similarly, by the concept of neighboring leads — the hyperacute T wave in lead V6 is flanked by more-voluminous-than-they-should-be T waves in leads V4 and V5 — which makes for 3 consecutive leads showing hyperacute T waves in the lateral chest leads.
- BOTTOM LINE: In this patient with new chest pain and LBBB — no less than 8/12 leads in ECG #2 show ST-T wave abnormalities that suggest acute OMI. Cardiac cath confirmed 100% occlusion of the RCA, which was stented.
- NOTE: For those interested in a user-friendly approach to the ECG diagnosis of the Bundle Branch Blocks (including a 13-minute ECG Video and PDF download) — Please check out ECG Blog #282.
- Additional posts in Dr. Smith's Blog, in which I've commented on qualitative aspects of LBBB tracings include — the April 7, 2019 post — the December 16, 2019 post —
- I thought the downsloping ST segments with biphasic terminal T waves in each of the inferior leads looked unusual — but nonspecific. From this single tracing — I couldn't tell if this was a new or old finding.
- The 1 lead in ECG #1 that most caught my eye was lead V5. As per the RED arrow in this lead — there is J-point ST depression with ST segment coving and T wave inversion. On the one hand — I thought this potentially could represent an acute finding. On the other hand (as suggested by enclosure within the dotted BLUE rectangle) — this might simply be a "transitional lead" finding — as ST-T wave morphology in lead V5 appeared to be intermediate between the very large, upright ST-T wave in lead V4 — and the deep ST-T depression in lead V6.
- BOTTOM LINE: I was uncertain if the ST-T wave in lead V5 did or did not represent an acute finding. That said — I saw no acute ST elevation in ECG #1, so there was no indication of an acute OMI on ECG. Subsequent astute analysis by Dr. McLaren made it apparent that the infarct in today's case was a NOMI, and not the result of acute coronary occlusion.
======================
- Virtually all patients with complete LBBB have some form of underlying heart disease. As a result — statistically, patients with LBBB make up a high-prevalence population for LVH.
- The presence of very deep S waves (ie, >25-30mm) in one or more anterior leads (ie, V1, V2 and/or V3) is highly correlated with LVH in patients with LBBB. LVH was almost certainly present in the TOP tracing in Figure-1.




-USE%20copy.png)





-USE.png)
-USE%20copy.png)




-USE.png)