Written by Willy Frick
A man in his 70s with a history of HFrEF and sick sinus syndrome s/p dual chamber pacemaker placement was admitted for overnight observation following outpatient placement of a mitral valve clip. The procedure note indicates uncomplicated clip placement. The next morning, the following ECG was obtained.
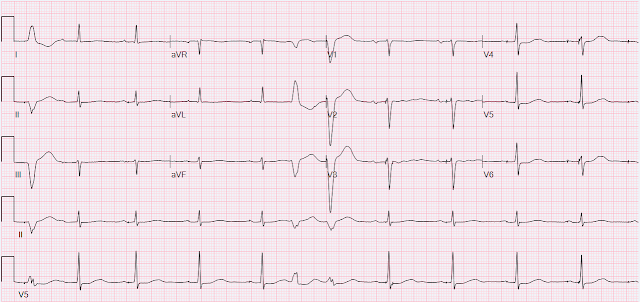
The tracing shows sinus rhythm with PVCs and non-specific ST&T wave abnormality. But there is something more important to notice, which is the pacer spikes. They do not make sense. Some of them are in the middle of or after P waves, and there's even one that falls at the end of the QRS! Here is the V5 rhythm strip blown up with numbered pacing spikes.
Comparing spikes 2, 3, 4, and 5 for example, we see that they all very clearly occur at different times in the cardiac cycle. Spike 2 occurs at the conclusion of the P wave, spikes 3 and 4 occur near the peak of the P wave, and spike 5 occurs just before the onset of the QRS. This is unambiguous evidence of pacemaker malfunction. When it comes to malfunction, we often sort into two categories (although there are other things that can go wrong with pacemakers).
"Failure to sense" occurs when the pacemaker does not recognize native function appropriately, and results in excess pacing spikes. Imagine the pacemaker is set at a minimum rate of 60. If it does not sense a native rhythm (perhaps from lead fracture or displacement), it will attempt to pace at 60 beats per minute, which can cause unnecessary pacing spikes interrupting normal cardiac function. As an analogy, imagine a faulty detector at a railroad crossing which fails to sense the oncoming train, and therefore leaves the gate open allowing cars to drive across the tracks into the oncoming train.
"Failure to capture" occurs when the pacemaker delivers an impulse that fails to actually depolarize the myocardium. This can cause pauses where there is no native output and no paced output. Here, imagine setting your morning alarm clock, but turning the volume down as low as possible. The alarm is going off, but you're sleeping through it!
Atrial lead
This tracing clearly shows failure to sense in the atrial lead. Spikes 2, 3, 4, 5, 7, 8, and 9 all occur either during or immediately after P waves which should never happen. The lead should have detected native P waves and suppressed pacing. There may be failure to capture, but you cannot be certain because all the pacing spikes occur at times when the atrium is either already depolarizing or still refractory from the prior depolarization.
Ventricular lead
This tracing may also show failure to sense in the ventricular lead. Spike 6 occurs at the conclusion of the QRS. The ventricular lead should have sensed a native QRS complex and suppressed pacing, but it did not, and therefore paced inappropriately. (For the EP enthusiasts, this may actually instead be due to Ventricular Safety Pacing, which is beyond the scope of this post.) Spike 10 actually shows a native/paced fusion complex (best appreciated in leads V4 and V6) which proves that this lead is still capable of pacing.
The finding of new pacemaker dysfunction after an invasive procedure should have prompted immediate investigation, but it went unnoticed. The final cardiologist overread was "Sinus rhythm with occasional AV dual-paced complexes and with occasional premature ventricular complexes," with no comment on pacemaker malfunction.
The case continues...
The patient was discharged the morning after the procedure. That evening when he arrived home, he had an unexpected fall while getting out of the car, blood pressure was reported to be 88/53. The patient and his wife suspected this was due to withholding of his midodrine and fludrocortisone during the hospitalization for his procedure. He had another unexplained fall the next day. He contacted the cardiology clinic to say that he felt "very tired with no energy" since the clip placement.
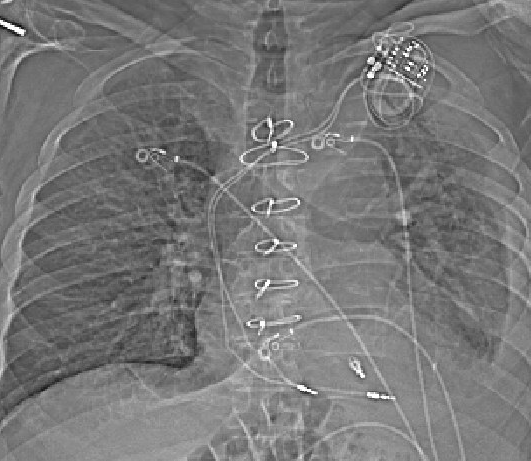
A few days after that, he was hospitalized for an unrelated reason, an abdominal infection. On the day of hospitalization, he had a CT scan. Beam hardening artifact limits ability to evaluate lead position on the actual CT, but the scout image is shown below:
This shows sinus rhythm at a rate of 87. There are occasional pacing spikes, but they are not causing any depolarization. They cannot be causing atrial depolarization (since they occur after the P waves), and they cannot be causing ventricular depolarization because all the QRS complexes are identical.
Over the next few weeks, the patient continued to complain of light-headedness several times per week. He had routine follow up for the mitral valve clip approximately 1 month after the procedure, and the note indicated recognition of atrial lead dislodgement on the CT scan from the hospitalization a few weeks prior.
The patient underwent an invasive procedure (the mitral valve clipping) which required traversing the right atrium and interatrial septum. It appears that during this procedure, his atrial lead was accidentally dislodged. The following day, he had clear ECG evidence of pacemaker malfunction that went unrecognized. He suffered from symptomatic bradycardia and falls for over a month and was seen by another cardiologist specifically for presyncope, and still the problem went unrecognized despite ongoing ECG evidence of pacemaker malfunction, and imaging proving lead dislodgement.
- Inspect the pacer spikes on the ECG to verify that they make sense. The ECG machine is particularly bad at complex rhythm analysis and pacemaker dysfunction.
- In patients with pacemakers, the chest x-ray contains a lot of information (lead configuration, type of device PPM vs ICD, positioning and dislodgment)
- Orthostatic hypotension is a common cause of syncope, but it is not the only cause of syncope, and other causes should be adequately ruled out.
- "Failure to sense" may result in excessive pacing.
- "Failure to capture" may result in inappropriate pauses without pacing.
MY Comment, by KEN GRAUER, MD (1/13/2024):
- We are not always provided with information as to whether the patient whose ECG we are looking at has a pacemaker. Sometimes, it is immediately obvious from the ECG we are given, that pacemaker spikes with appropriate sensing and capture are present. But at other times — Have YOU ever wondered?
- We are not always fortunate to receive a high-quality tracing (as we have in today's case). And the ECG we are given is often not essentially free of artifact (as it is have in today's case).
- We often do not have a baseline ECG for comparison.
- And, optimal filter settings for detecting pacemaker spikes are often not selected (with notation of which filter setting was used often not included in the ECG picture transmitted via the internet).
- Being told this patient did have a pacemaker — was extremely helpful in convincing me that (as per Dr. Frick) — that there were multiple atrial pacing spikes occurring at all points in the cardiac cycle, without apparent relation to the timing of sinus-conducted QRS complexes.
- KEY Point: The amplitude of many of these atrial pacing spikes is tiny — and could easily be overlooked if ECG monitoring was limited to a single-lead rhythm strip, for example of lead V1! (Note from the simultaneously-occurring YELLOW and RED double-arrows in Figure-1 — that there is no indication at all that atrial pacing spikes seen in lead V5 produce a spike in lead V1).
- Even in lead II (which together with lead V1, are generally viewed as the best leads for visualizing atrial activity) — the YELLOW double-arrow in lead V5 that clearly identifies an atrial pacing spike immediately after the QRS of beat #8 — provides no indication in the simultaneously-recorded long lead II rhythm strip of an atrial pacing spike.
- The long lead II rhythm strip in today's case does provide indication of atrial pacing elsewhere — but isn't the amplitude of these atrial pacing spikes in the long lead II tiny? Do YOU really think you would be able to identify these tiny atrial pacing spikes IF: i) You were not told that the patient had a pacemaker? — ii) You did not have a baseline tracing? — and — iii) The ECG you were given was from a smart phone photo from a patient with a certain amount of movement and/or baseline artifact?
-USE.png) |
| Figure-1: I've labeled the initial ECG in today's case. |
- Different settings are typically used for ECG recording, depending on whether emphasis is placed on rhythm determination vs diagnostics (with focus for diagnostics being placed on interpreting 12-lead waveforms).
- Greater filtering is generally used in monitor mode, with a common frequency setting being between 0.5 Hz and 40 Hz (Hz = Hertz). Doing so has the advantage of minimizing artifact and baseline wander that may affect rhythm interpretation.
- KEY Point: Because pacemaker spikes tend to be a high frequency signal — they are often effectively filtered out by a monitor mode setting of 0.5-to-40 Hz. When this is the filter setting used — pacer spikes may simply not be seen on the ECG (ie, Ventricular pacing can easily be misdiagnosed in such cases as LBBB or as AIVR — Johnson Francis, 2017).
- In contrast to monitor mode — a broader passband (typically from 0.05 Hz to 150 Hz) is recommended for diagnostic mode, for which emphasis is on optimally accurate ST segment analysis.
- The generally recommended frequency setting for 12-Lead ECG Diagnostic Mode ECG interpretation in adults is 0.05-to-150 Hz. An even higher upper frequency limit may be used in infants or young children. If muscle artifact is severe, one may need to compromise with a narrower range setting of 0.05-to-100 Hz (or 0.05-to-40 Hz) — but the recommended range 0.05-to-150 Hz should be tried first.
- KEY Point: If compromise in filter settings is needed, then at least be aware that there may be less than optimal fidelity for ST segment analysis. This explains why monitoring lead rhythm strips should not be used for ST segment analysis. One may be alerted to possible (or even probable) ST segment elevation or depression based on changes seen on a rhythm strip — but when clinically important — this must always be verified on a 12-lead tracing.
- For illustrative purposes — I have taken the liberty of adding in the optimal frequency filter range for a 12-lead ECG (ie, 0.05-to-150 Hz) — standard paper speed in the U.S. (ie, 25 mm/sec) — and standard voltage calibration (ie, 10 mm/mV).
- Note how the change in filter settings (presumably from 0.05-40 Hz in the TOP monitoring leads in Figure-2 — to 0.05-150 Hz in the simultaneous 12 lead ECG) — dramatically altered the amount of chest lead ST elevation! The only way to determine if ST elevation or depression on a monitoring lead is real — is by immediately obtaining a 12-lead ECG.
- KEY Point: Consider the artifact that is especially prominent in lead V2 of Figure-2 — but which is also present at lower amplitude in virtually all of the limb leads. Would YOU be able to see low-amplitude atrial pacing spikes if similar artifact was present in all leads on the tracing?
 |
| Figure-2: What the monitor shows (TOP) — and what a simultaneously-obtained 12-lead ECG on this patient shows (See text). |
- Confession: I fully acknowledge that I was many years into my career as an “ECG enthusiast” until I finally began to pay attention to these settings. This is important — because use of a different filter setting can be the reason why ST segment deviation (elevation or depression) may give false impression of a difference in ST elevation or depression changes between serial ECGs done on the same patient!
- In Today's CASE: A low-pass filter setting significantly less than 150 Hz might result in not seeing pacer spikes at all on the ECG.
- P.S.: For another example regarding selection of an optimal filter setting — See My Comment at the bottom of the page in the June 3, 2023 post in Dr. Smith's ECG Blog — in which I illustrate the effect of filter settings for detection of epsilon waves in a patient with of ARVC/D (Arrhythmogenic Right Ventricular Cardiomyopathy-Dysplasia).



No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.