Written by Jesse McLaren, with edits from Smith
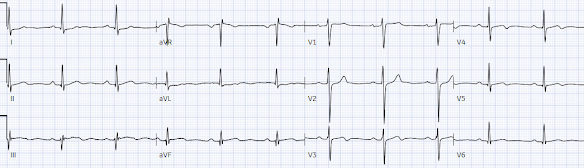
A 30 year old with a history of diabetes presented with two days of intermittent chest pain and diaphoresis, which recurred two hours prior to presentation. Below is ECG #1 at triage. Are there any signs of occlusion or reperfusion?
There’s normal sinus rhythm, normal conduction, normal axis, normal R wave progression and normal voltages. There’s mild inferior ST elevation in III that doesn’t meet STEMI criteria, but it’s associated with ST depression in aVL and V2 that makes it diagnostic of infero-posterior Occlusion MI (from either RCA or circumflex)– accompanied by inferior Q waves of unknown age. There are also subtle biphasic T waves in V3-4 of unclear significance (this can be seen in anterior or RV reperfusion, but this usually does not accompany infero-posterior occlusion).
Just so you know this ECG interpretation is not done by the retrospectoscope:
I sent it to Dr. Smith without any information, and he immediately responded: "Infero-postero-lateral OMI"
The ECG had a computer and final cardiology interpretation of “possible inferior infarct, age undetermined”, because of Q waves. It was signed off by an emergency physician as “STEMI negative” because it did not meet STEMI criteria. So the patient waited to be seen. First troponin I returned at 150 ng/L (<26 in males and <16 in females) and ECG #2 was performed, with the patient painfree. What do you think?
Now the ST elevation in III with reciprocal change in aVL is resolving, and V2 shows normalization of ST segment and slightly bigger T wave – so there’s been infero-posterior reperfusion. And the biphasic T waves in V3-4 persist.
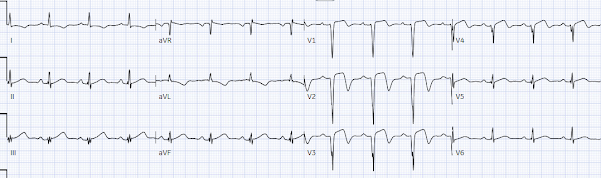
The patient was seen by an emergency physician 5 hours after arrival, reporting no symptoms, with repeat trop down to 125 ng/L and ECG #3. What do you think?
ST segment has risen again in III and there are bulkier inferior T waves, with reciprocal ST depression in aVL and ST depression in V2. But now there’s also been a loss of anterior R waves with new Q waves, and the previously biphasic T waves in V3-4 are now upright (pseudonormalization). Moreover, T-waves in V3 and V4 are now bulky, suggesting they are hyperacute. So despite a declining initial troponin and resolution of symptoms, the ECG shows reocclusion with OMI affecting inferoposterior plus anterior walls. But it still doesn’t meet STEMI criteria, so the patient was referred to cardiology as ACS.
10 hours after arrival the patient was assessed by cardiology and had ECG #4:
The ST elevation and reciprocal ST depression in aVL has improved and T waves are no longer hyperacute. The transiently upright T waves in V3-4 have inverted again, and now there’s T wave inversion across the anterolateral wall. Now the main pattern is anterolateral reperfusion, most likely from proximal LAD
13 hours after arrival the patient complained chest pain and ECG #5 was obtained, and repeat troponin was 11,000 ng/L.
There’s further loss of precordial R waves (in fact, profound Q-waves), with increasing ST segment in V2 but ongoing reperfusion T wave inversion anterolateral. Recurring symptoms suggest reocclusion, with an ECG between reperfusion and reocclusion. The chest pain was refractory to nitro so the cath lab was activated: 100% proximal LAD and 99% mid circumflex occlusions.
Peak troponin was 15,000 ng/L, and echo found preserved EF but
akinetic apex and hypokinesis of anterior and inferior walls. Next day ECG #6:
Discharge ECG #7 a few days later :
Dual OMI, and dueling OMI
STEMI is usually attributed to one infarct-related artery that becomes completely and irreversibly occluded, producing regional ST elevation that exceeds STEMI criteria. But this framework is proven to be unreliable, and the underlying pathology of Occlusion MI is much more complicated and dynamic: there can be little or no ST elevation but multiple other signs of OMI, and this dynamic state can fluctuate between spontaneous reperfusion and spontaneous reocclusion.
In addition, there are a number of other scenarios that complicate OMI and ECG interpretation:
· the occlusion of one infarct-related artery can affect other territories based the site of occlusion. For example anterior and inferior ST elevation can be caused by proximal RCA or distal/wraparound LAD occlusion
· the occlusion of one infarct-related artery can occlude collaterals that were serving another territory with chronic total occlusion
· the occlusion of one infarct-related artery can induce subendocardial ischemia in another that had critical stenosis. For example, inferior OMI with concomitant critical stenosis produces a combined pattern (Aslanger’s pattern) with inferior STE and subendocardial ischemia
· occlusion of two infarct-related arteries simultaneously ("co-culprits")
In this case there were two infarct-related arteries. This complicated ECG interpretation because 1) there were two different patterns of Occlusion, 2) neither of which ever met STEMI criteria, 3) they were reperfusing/reoccluding at different times, and 4) had different effects on some of the same leads. For example aVL is a high lateral lead which is reciprocal to the inferior wall: inferior OMI can cause inferior ST elevation/hyperacute T with reciprocal ST depression in aVL (ECG #1/3), while lateral reperfusion can cause primary T wave inversion in aVL (ECG#4-7) with reciprocally tall inferior T wave. Similarly, the anterior lead V2 is reciprocal to the posterior wall: posterior OMI can cause reciprocal ST depression in V2, while anterior reperfusion can cause primary T wave inversion in V2.
In other words, there was not only dual OMI, but they were dueling – each going back and forth between occlusion and reperfusion, with clashing ECG patterns that sometimes favoured one and other times favoured the other:
· ECG #1: circumflex OMI dominated, with subtle LAD reperfusion
· ECG #2: reperfusion of circumflex, LAD still reperfused
· ECG #3 reocclusion of circumflex and LAD
· ECG #4-7: reperfusion of LCX and LAD, the latter of which dominated
Take away
1. Young people can have acute coronary occlusion.
2. Symptoms don’t always correlate with coronary artery occlusion/reperfusion: resolved symptoms but ongoing ECG signs of occlusion requires reperfusion.
3. STEMI criteria is unreliable and leads to delayed reperfusion: neither of these occlusions ever meet STEMI criteria, but there were multiple other diagnostic signs of occlusion across the entire QRS-T complex – including loss of R waves and new Q waves, subtle ST elevation, reciprocal ST depression, pseudonormalization and hyperacute T waves.
4. STEMI criteria also ignores spontaneous reperfusion at risk for reocclusion. The question is not whether the ECG meets STEMI criteria, but whether the patient has OMI, including reperfused OMI at risk for reocclusion.
5. Troponin is a delayed marker of ischemia that is unreliable early in occlusion, or in spontaneous reperfusion at risk for reocclusion: here the first trop was only 150 and declined on repeat, only to rise to a peak of 15,000.
6. The angiogram can highlight occlusions at the moment of the procedure, but it’s serial ECGs which tell the story of occlusion and reperfusion.
7. OMI ECG changes can be complicated by site of occlusion, compromised collaterals to chronic total occlusions, concomitant critical stenoses, or co-culprits – resulting in combined ECG patterns with dynamic changes based on underlying reperfusion/reocclusion.
- Errors in today's case resulted in a 13-hour delay. It wasn't until the 5th ECG was obtained (apparently done only because the patient's chest pain had returned) — that the by now markedly elevated Troponin and by now obvious ECG changes finally convinced providers of the need for cardiac cath.
- Unfortunately, significant cardiac damage had already been done. This might have been minimized had the abnormalities been picked up earlier.
- The stuttering course of chest pain in today's case (ie, intermittent over 2 days — and then returning ~2 hours prior to ED arrival) — is relevant for optimal management of today's patient.
- Even clinicians still "stuck" on the STEMI paradigm need to accept that acute coronary occlusion is often a dynamic evolving process, instead of a single "static" event. The "culprit" artery acutely occludes — but then it sometimes spontaneously reopens — and, occasionally continues for a period of time to spontaneously reopen and reclose a number of times, until eventually a permanent status is reached. The stuttering course of chest pain in today's case should have suggested this dynamic evolution — especially after seeing the initial ECG!
- Abnormal ST-T waves are actually present in 10/12 leads in ECG #1. While the ED physician was correct in saying that this initial ECG "did not meet STEMI criteria" — I feel there are ST-T wave changes in 3 leads that can not be ignored.
- I was not initially convinced that the subtle ST elevation in leads III and aVF was significant — until I saw the flat ("shelf-like") ST depression in lead aVL. While not necessarily acute — given the stuttering history of chest pain over the past 1-2 days, the ST-T wave appearance in lead aVL has to be interpreted as a reciprocal change from presumed recent infarction until proven otherwise.
- In the context of lead aVL — the other high-lateral lead ( = lead I) shows a lesser degree but-still-significant amount of flat ST depression.
- There is no way that the flat ("shelf-like") shape of ST depression in lead V2 is normal. Especially in association with an already surprisingly tall R wave in lead V2 — this (as per Dr. McLaren) is diagnostic of posterior OMI until proven otherwise. (Remember that normally — there is usually slight, upward sloping ST elevation in lead V2 — and virtually never ST depression).
- In a patient with intermittent chest pain — the straightened ST segment take-off in lead V3, with terminal T wave inversion (RED arrow) is clearly of concern. This ST-T wave appearance is not expected with posterior OMI — but instead suggests the possibility of either Wellens' Syndrome (if chest pain has resolved) — or — reperfusion following recent LAD occlusion.
- In support of significance for the abnormal ST-T wave appearance in lead V3 — is the subtle-but-real terminal T wave inversion in neighboring leads V4 and V5.
- A final abnormal finding in the chest leads — is that the upright T wave in lead V1 is taller than the upright T wave in lead V6. Although this is a nonspecific finding — it is often associated with ischemia.
- While millimeter criteria for a STEMI are not met in ECG #1 — and while ST-T wave changes in most leads in the initial ECG are not definitive — the ST-T wave appearance in 3 leads ( = leads aVL — V2 — V3) can not be ignored as indication that an acute event may have recently occurred.
- At the very least — the initial ECG should have been repeated within 10-20 minutes. Seeing even subtle ST-T waves changes (such as less ST depression in lead V2, with an increase in T wave positivity in this lead — as was eventually seen when the 2nd ECG in today's case was obtained) — would be evidence of dynamic change that confirms reperfusion from recent posterior OMI.
- The 1st troponin did come back elevated (150 ng/L). Given the worrisome history and the initial ECG — this result should have been enough to merit prompt cath.
- To Emphasize: — I had no idea from the initial ECG that there were 2 "culprit" arteries in today's case. Had I known this — it would have explained some of the subtle contrasting ECG findings. But the point is that we do not need to know the anatomy in order to recognize that in a patient with new chest pain — the ST-T wave appearance in leads aVL, V2 and V3 of the initial ECG can not be ignored until we have actively ruled out a recent (or still ongoing) acute event.
-USE%20copy.png) |
| Figure-1: I've labeled the 3 leads of most concern in the initial ECG from today's case. |






No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.