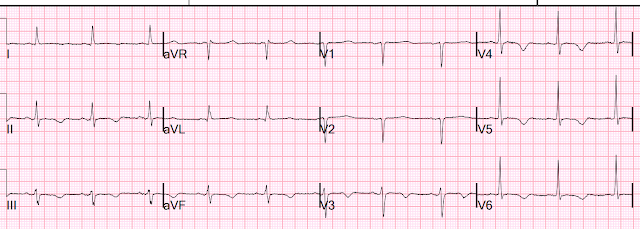
I was shown this ECG and told that the patient is suffering from another bout of chronic pancreatitis. I was told there was no chest discomfort or SOB.
I said "this looks like takotsubo".
With this ECG and the presumptive diagnosis of pancreatitis, takotsubo stress cardiomyopathy is by far most likely. It is possible that it is due to ACS, but the bizarre diffuse T-wave inversions with a very long QT are nearly pathognomonic of takotsubo. (They can also be seen in apical hypertrophic cardiomyopathy, but this patient did not have any such history and previous ECGs were different)
If it is takotsubo, there is usually apical ballooning on echo.
If it is ACS, it would need to be anterior and inferior ACS, and there will often be corresponding wall motion abnormalities. Apical ballooning can appear very much like anterior + inferior WMA, so echo does not necessarily distinguish the two.
However, absence of any WMA is very unusual for takotsubo, but not unusual for Non-OMI.
POCUS echocardiogram was done but was NORMAL!
Thus, we must strongly suspect that this is myocardial infarction (takotsubo is classified as non-MI myocardial injury)
Electrolytes were normal and the only QT prolonging medication on the list was ondansetron. We do not know how much ondansetron he was taking.
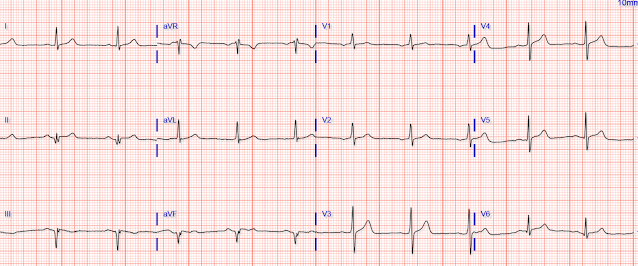
The first troponin returned elevated at 271 ng/L. There were previous troponins from 2 visits about 4 and 5 weeks before.
Looking back, he presented several times recently:
------------------------------- 4 weeks prior, visit 3 (4 serial values)--------------------// --5 wks prior, visit 2
1 value
Here is the history of this presentation (presentation 5):
The patient had 9/10 epigastric pain that radiated to the back for almost 24 hours associated with alcohol intake, with nausea and vomiting, and it feels similar to previous chronic pancreatitis.
But he also has substernal chest burning with SOB. Was he having pancreatitis AND acute MI?
Let's go back and look at previous visits.
Presentation 1
__________________________________
Presentation 2, abd pain
Abd pain again on presentation 3
Diagnosis at this visit was chronic pancreatitis, with troponins at 7, 8, 8, and 8 ng/L; he was admitted for pancreatitis.
Presentation 4. Altered ms, no ekg, no troponins
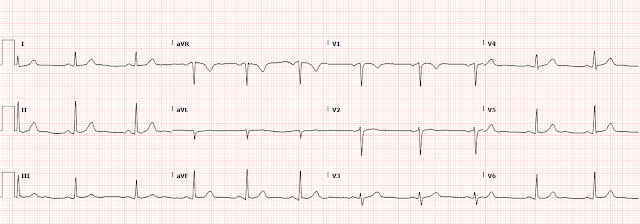
Presentation 5 (the one we are presenting, first ECG above at the top, and shown again here)
66 hours
90 minutes after PCI
Formal Echo also was nearly normal, with a possible suggestion of "apical takotsubo"
So he underwent angiogram:
LMCA: No angiographic significant obstructive disease.
LAD: Type III LAD is noted. Severe calcification is present. LAD distal to large LADD1 has 80%. Proximal to LADD1 has a 60% assessed via iFR 0.84. Pre procedure TIMI III flow was noted for proximal lesion.
LCx: Proximal LCX has indeterminate , 60% disease assessed via FFR 0.80, iFR 0.98
OM1 large with a large lateral segment
Distal LCX before the LPL1 has a 80-90% stenosis
Lesion on Prox CX: The Proximal segment of the RCA has 60-70% disease. The Distal segment of the RCA has 90% disease. RPDA diffusely diseased.
Cardiology thought process here:
EKG shows deepening T wave inversions and prolonged QT throughout first hospital day. DDx for TWI and QTc prolongation includes stress CM (takotsubo), HOCM, intracranial bleed, and cardiac ischemia. TTE not entirely convincing of HOCM but cannot rule out apical HOCM. Stress CM also cannot rule out. Reviewing previous imaging, he does have extensive calcifications of his coronary arteries. Angiogram was completed and revealed 3 vessel coronary artery disease involving the LAD. After discussion with patient and CT surgery decision was made to pursue PCI. Stent was placed in the proximal and mid distal LAD 7/08.
Successful PCI to LAD/Diagonal with adjunctive rotational atherectomy, excellent angiographic result with residual apical LAD stenosis that was appropriate to defer. Due to procedural length and probable LAD "culprit", will defer treatment of Cx and RCA. Will need 12 months of clopidogrel-based DAPT, high-intensity statin therapy, oral beta blockade, and ACEi - if BP tolerates - in the setting of "anterior unstable angina". Smith comment: This last bit is clearly a mis-statement, as this ACS is acute MI, not unstable angina.
Learning Points:
1. If you think it is takotsubo, but the echo is not consistent, then think ACS.
2. Patients may have 2 pathologies at the same time (Hickum's dictum). This patient definitely had pancreatitis on at least one visit, but also had either or both of takotsubo AND ACS.
3. Takotsubo and Acute MI may be very difficult to differentiate on ECG, whether they present as ST Elevation (OMI vs. OMI mimic) or with bizarre T-wave inversion (Non-OMI vs. Non-OMI mimic).
4. They may even be difficult to differentiate by echo, and even by angiogram (was it a culprit or not? The angiographer thinks so, but I think he was not certain)
5. Takotsubo should have obvious diffuse wall motion abnormalities resulting in apical ballooning.