Written by Jesse McLaren, with comments from Smith
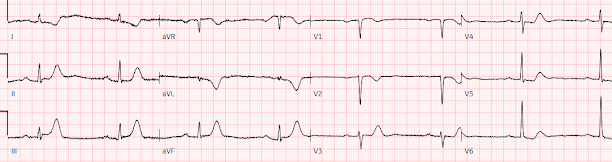
An 85 year old with a history of CAD presented with 3 hours of chest pain that feels like heartburn but that radiates to the left arm. Below is the ECG. What do you think?
There’s sinus bradycardia, first degree AV block, normal axis, delayed R wave progression, and normal voltages. There’s minimal concave ST elevation in III which does not meet STEMI criteria, so this ECG is "STEMI negative". But there are multiple other abnormalities that when combined are diagnostic of OMI and predictive of RCA occlusion:
- sinus bradycardia, which is common in RCA occlusion
- inferior hyperacute T waves (broad based, symmetric, tall relative to the QRS)
- reciprocal ST depression and T wave inversion in aVL (and I), which is highly specific for inferior OMI
- primary anterior ST depression, which is posterior OMI until proven otherwise
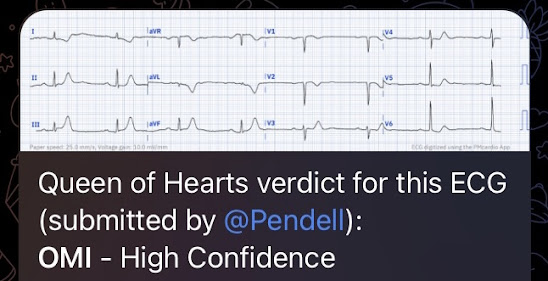
Here's the interpretation of the PMcardio AI trained in identifying OMI:
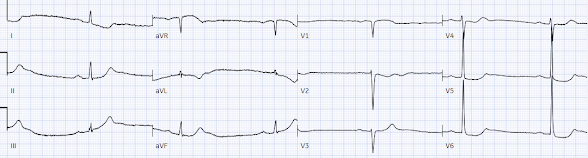
Below is the old ECG, showing the first degree AV block, delayed R wave progression and some of the precordial ST depression is old especially in the lateral leads. But the bradycardia and the infero-posterior OMI is definitely new:
 Smith: this also has many abnormalities suggestive of ischemia: many leads have ischemic appearing ST depression
Smith: this also has many abnormalities suggestive of ischemia: many leads have ischemic appearing ST depression
The emergency provider followed the sequential steps of the current paradigm:
1. Use STEMI criteria to identify acute coronary occlusion: the ECG was STEMI negative
2. Use troponin to rule out non-STEMI: two high sensitivity troponin I performed two hours apart were 4 and 16 ng/L, both in the normal range (upper limit of normal 16 in females and 26 in males). The assay was Abbott Alinity, which is very similar to Abbott Architect high sensitivity troponin I. See analysis below.
3. Arrange follow up for chest pain patients who are “STEMI negative” with “normal troponin”: the patient was referred to outpatient cardiology
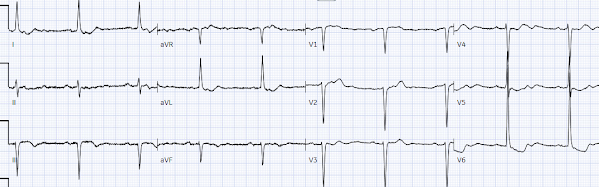
But 6 hours later the patient returned with recurrent chest pain:
Again diagnostic of infero-posterior OMI, though this time it does STEMI criteria, albeit barely. The cath lab was activated.
A repeat ECG was done on way to cath lab:
"STEMI negative" again. Hyperacute T waves are deflating, suggesting reperfusion but there is still reciprocal change in I/aVL and ST depression in V2, and the bradycardia is worse. On angiogram there was a 90% RCA occlusion. Troponin rose from 600 to 17,000 ng/L.
Discharge ECG showed resolution of bradycardia, inferior reperfusion T wave inversion, and baseline precordial ST depression.
Take home
1. As the new ACC consensus states (citing the work of Smith/Meyers), "The application of STEMI ECG criteria on a standard 12-lead ECG alone will miss a significant minority of patients who have acute coronary occlusion. Therefore, the ECG should be closely examined for subtle changes that may represent initial ECG signs of vessel occlusion, such as hyperacute T waves...or ST-segment elevation <1 mm, particularly when combined with reciprocal ST-segment depression, as this may represent abnormal coronary blood flow and/or vessel occlusion."
2. Using troponin for acute coronary occlusion is like relying on a rear-view mirror to navigate a car pile-up: it shows wreckage behind you that has already happened, but can’t see the road ahead and can give false reassurance when there's a head-on collision happening in real time. It’s common for acute coronary occlusion to present with troponin in the normal range, and the initial rise can’t predict the final damage. Even if the troponin on the first visit had been higher there still would have been delayed reperfusion because it would have been diagnosed as "non-STEMI"
3. Using risk stratification tools like HEARTS or EDACS may have avoided the initial discharge, but shouldn’t be used if the ECG is already diagnostic of OMI. (See this other post: Chest pain, a ‘normal ECG’ a ‘normal trop’, and low HEART and EDACS score: discharge home? Stress test? Many errors here.) There’s also a hazard of relying on troponins that are in the normal range but above the level of detection. As this study from Dr. Smith concluded: “measurable hs-cTnI concentrations less than or equal to sex-specific URLs have important prognostic implications. Our findings underscore the importance of recognizing cTn as a continuous variable, with the higher the cTn, the higher the probability of MACE. We caution against the clinical use of the terms normal or negative among such patients.” (Clinical features and outcomes of emergency department patients with high-sensitivity cardiac Troponin I concentrations within sex-specific reference intervals.)
Smith comments on troponin:
- For ease of comparison in Figure-1 — I’ve reproduced the first 3 ECGs that were done in today’s case.
- Considering that today's patient presented with new CP (Chest Pain) — the initial ECG is already diagnostic of an acute event until proven otherwise.
- As noted by Dr. McLaren, compared to the prior tracing — there are a number of new ST-T wave changes in ECG #1.
- There is no notation of whether CP was still present at the time ECG #1 was obtained (and if so, whether CP was increasing, remaining constant, or decreasing). Without this information — it is impossible to understand if the acute-looking ST-T wave changes in ECG #1 might indicate ongoing acute occlusion vs spontaneous reperfusion vs spontaneous reocclusion.
- In addition to the above missteps — the Troponin Delta (ie, the increase in Troponin from 4-to-16 ng/L) that was interpreted as “negative” — is not a "normal" result (as discussed in detail by Dr. Smith). Therefore, even without the acute ECG changes seen in this case — full evaluation of this patient would be needed.
- I favor picking one of the 2 tracings that you are comparing — and systematically interpreting that tracing in its entirety before you look at the 2nd tracing.
- When comparing a current tracing with a prior ECG — we ideally should know the circumstances under which the prior tracing was done (ie, Was the patient stable and without symptoms? — or — Was the prior tracing obtained during chest pain or soon after an infarction?). Unfortunately — We do not know the circumstances under which the prior tracing in today's case was recorded.
- Are ECG parameters in the 2 tracings you are comparing similar? (ie, Is there a change in the frontal plane axis? Is R wave progression similar? Is the heart rate and rhythm in the 2 tracings the same?). Significant change in any of these parameters may result in ST-T wave changes that are not the result of ischemia or infarction.
- As per Dr. McLaren — there is marked sinus bradycardia and arrhythmia (ie, heart rate in the 40s) — with 1st-degree AV block (PR interval ~0.23 second).
- Regarding other intervals — the QRS is narrow — and the QTc is probably normal given the slow rate. The frontal plane axis is normal (about +70 degrees). There is no chamber enlargement.
- There are no significant Q waves (ie, The QS in lead V1 is not abnormal per se). A tiny-but-present initial r wave is seen in lead V2 — with this R wave progressively increasing across the precordium. Transition (where the R wave becomes taller than the S wave is deep) — is slightly delayed (to between leads V3-to-V5).
- ST segments are straightened in multiple leads. In the inferior leads, this is associated with slight J-point ST elevation and clearly hyperacute T waves (that are disproportionately tall, "fat" at their peak — and wider than expected at their base).
- Reciprocal changes (ie, a mirror-image opposite ST-T wave picture) — are seen in lead aVL, and to a lesser extent in lead I. Considering how tiny QRS amplitude is in these high-lateral leads — these have to be considered acute changes until proven otherwise!
- In the Chest Leads — ST-T wave changes are equally concerning. There is ST segment coving with T wave inversion in leads V1,V2. We see a distinct straightening with downsloping of the ST segment in leads V3-thru-V6. This is followed by terminal T wave positivity in these leads — with T waves in leads V3,V4,V5 being clearly "hypervoluminous" ("fatter"-at-their-peak and wider-at-their-base than they should be — as well as disproportionately tall in leads V3,V4 considering R wave amplitude in these leads).
- IMPRESSION of ECG #1: As per Dr. McLaren — Especially in view of the marked bradycardia, the above ECG findings are diagnostic of acute infero-postero OMI until proven otherwise! The ST segment coving in leads V1,V2 suggests possible acute RV involvement — with acute occlusion of the RCA as the presumed "culprit" artery. Given the history of new chest pain — prompt cath is clearly indicated on the basis of this initial ECG.
- Several differences in ECG parameters make comparison of ECG #1 with ECG #2 challenging. These include: i) The much faster heart rate in the prior tracing; and, ii) Little change in the frontal plane axis — but clearly increased QRS amplitude in the prior tracing.
- Although straightening of ST segments is not a new finding in ECG #1 — there should be no doubt that the subtle ST elevation in leads III and aVF is real — since if anything, there was slight ST depression in these leads on the prior tracing. Similarly, the hyperacute T wave appearance in these inferior leads is markedly increased in ECG #1.
- Reciprocal ST-T wave depression with T wave inversion is similarly markedly accentuated in leads I and aVL of ECG #1.
- Although ST segment straightening with prominent T waves was present in the prior tracing — lead-by-lead comparison suggests that the T waves in leads V3-thru-V6 in ECG #1 are relatively taller (considering QRS amplitude in each respective lead) — and definitely "fatter"-at-their-peak and wider-at-their-base (ie, more hyperacute) than they were in the prior tracing.
- IMPRESSION: In this 85-year old patient with new chest pain — comparison of the prior tracing with ECG #1 should remove all doubt about the acuity of ECG changes on this initial tracing. Prompt cath is clearly indicated — especially in view of the worrisome bradycardia in ECG #1. The patient should not have been sent home.
- Comparison of ECG #3 with the initial ECG done 6 hours earlier — and with the "baseline" (prior) tracing, provides insight into the sequence of ECG changes correlated to patient symptoms.
- There is now definite ST elevation in all 3 inferior leads in ECG #3 — in association with T-QRS-D (Terminal-QRS-Distortion — as the S wave in leads III and aVF has been lifted from the baseline) + an even greater increase in relative size of the hyperacute inferior T waves (The T waves in leads III and aVF now tower over the R waves in these leads — whereas they were approximately the same height as the R waves in ECG #1).
- Reciprocal ST-T wave depression/T wave inversion in high-lateral leads I and aVL has increased a comparable amount to the inferior lead ST elevation.
- In contrast — ST-T wave changes look less prominent in ECG #3 than they were on the initial tracing. The evolution of sequential ECG changes during an acute ongoing event is not always homogeneous.
-USE.png) |
| Figure-1: Comparison between the initial ECG in today's case — with a prior tracing — and with the repeat ECG (done 6 hours after ECG #1). |





No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.