A 90 year old with a history of atrial fibrillation presented with two weeks of intermittent retrosternal chest pain lasting minutes. An hour prior to presentation it became constant and more severe, accompanied by nausea and general weakness, and the paramedics brought them to the ED as a code STEMI. Heart rate was in the 50s and other vitals normal. What do you think?
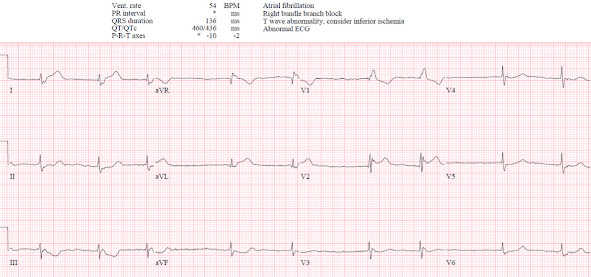
There’s atrial fibrillation, a right bundle branch block, normal axis and normal voltages. RBBB should produce secondary ST depression and T wave inversion in the anterior leads with the RsR’ (as it does in V1). But here in V2 there’s inappropriate ST elevation and upright T wave. There’s also mild STE in I/aVL, discordant to the slurred S wave, which can sometimes be seen in RBBB. But there are also down/up T waves in III/aVF which are reciprocal to what could be hyperacute T waves in I/aVL: compare the bulk of these T waves and their size relative to the QRS, compared with the normal sized T wave in V5-6.
So this could be RBBB with the “South African flag” pattern of injury in I/aVL/V2 with reciprocal change in III/aVF, corresponding to occlusion of the first diagonal artery[1]. The same pattern was seen on the ECG the paramedics performed, which initiated their STEMI transfer: note the concordant ST elevation in V2 with straightening of the ST segment.
Cardiology assessed the patient in the ED and noted: “RBBB with isolated 1.5mm STE concave up in V2, and borderline 1mm STD in III, not meeting STEMI criteria. Bedside echo grossly normal. Not a code STEMI based on ECG not meeting criteria and absence of regional wall motion abnormality on bedside echo.” So they cancelled code STEMI.
The emergency physician saw the patient, noted the cardiology opinion of normal regional walls and lack of STEMI, so looked for other causes of chest pain. Initial high sensitivity troponin I was 6ng/L (normal <16 for females and >26 for males). This does not rule out OMI in a patient with only one hour of chest pain: a quarter of patients with STEMI presenting within two hours of symptom onset have troponin less than 99th percentile [2]. But this could contribute to diagnostic momentum away from OMI. D-dimer and CXR were normal, but the pain continued so the patient received Tylenol and repeat troponin.
Two hours after arrival the patient was still in pain, repeat troponin was 50, and a repeat ECG was done.
Similar, but now there is the beginning of Q wave and convex ST segment in V2. The patient received morphine and waited for a CT chest to rule out dissection.
Six hours after arrival the CT chest was unremarkable but the patient was still in pain, and repeat trop was 200. So they received nitro and more morphine, CCU consult, and a repeat ECG:
Now they’ve totally lost their R waves in I/aVL and V2, confirming D1 infarct.
CCU consult noted “RBBB with ST changes in I/aVL and V2. Given lack of alternative causes and troponin elevation, most likely diagnosis is NSTEMI, so will admit for angiogram.” Refractory ischemia is an indication for immediate reperfusion according to guidelines, but nearly 95% of high-risk NSTEMI are not managed this way [3]. Eleven hours after arrival, repeat troponin was 1700. Thirteen hours after arrival the patient received more morphine and Tylenol for ongoing pain. Sixteen hours after arrival, repeat troponin was 18,000.
The patient was then taken for cath, which found a 95% D1
occlusion. Next day echo found corresponding “mid-distal anterior and lateral
walls and apical septum are severely hypo/akinetic”, with an EF of 40%, confirming
the initial bedside echo reading was unreliable. Peak troponin was 25,000, confirming
the initially normal troponin was unreliable. Discharge ECG showed Q waves
I/aVL and V1-3, with T wave deflation and inversion:
Discharge diagnosis was “NSTEMI”. This ensures that the initial paramedic transfer will be classified as a false activation rather than a false cancellation, and that the major delay to reperfusion (after troponin was already 18,000, despite initial troponin of 6) will be considered standard of care for NSTEMI rather than an opportunity for improvement.
Take away
1. Code STEMI cancellations should be reassessed and reclassified, not based on whether the ECG met STEMI criteria but whether the patient had OMI
2. STEMI criteria miss ECG and clinical signs of OMI, including first diagonal occlusion pattern, hyperacute/reciprocal T wave changes, acute Q waves, and refractory ischemia
3. Bedside echo can complement but should not replace ECG interpretation, because both have strengths and limitations
4.
An initial troponin in the normal range does not rule out OMI, and a rise in troponin is a delayed marker of OMI
5. Morphine is associated with delayed reperfusion
6. Refractory ischemia is an indication for immediate reperfusion
7. MIs should be classified as OMI/NOMI not STEMI/NSTEMI to learn from and avoid reperfusion delays
References
1. Littman. South African flag sign: a teaching tool for easier recognition of high lateral infarct. Am J Emerg Med 2016
2. Wereski et al. High-sensitivity cardiac troponin concentrations at presentation in patients with ST-segment elevation myocardial infarction. JAMA Cardiol 2020
3. Lupu et al. Immediate and early percutaneous coronary intervention in very high risk and high risk non-ST segment elevation myocardial infarction patients. Clin Cardiol 2022
===================================
MY Comment, by KEN GRAUER, MD (7/18/2022):
===================================
- Is the shape of the ST-T waves suggestive of acute occlusion?
- Is the size of ST-T wave changes proportionate, relative to QRS amplitude in the lead being looked at?
- Is the location of ST-T wave changes (elevation or depression) consistent with a defined anatomic area(s) — and — suggestive of an acute event?
-USE%20copy.png) |
| Figure-1: I've put the first 2 ECGs done in the ED together (See text). |
- Today's patient was both "high prevalence" and "high risk" for significant morbidity/mortality from acute MI. The patient was 90 years old — with a known history of AFib — and a worrisome account of chest pain over recent weeks that 1 hour before presentation became constant and much more severe.
- Adding to that risk (even before reviewing her ECGs) — is her slow AFib rhythm, with heart rate in the low 50s despite constant, severe chest pain.
- QRS voltage everywhere is reduced. Of itself, this is a potential risk factor — as in association with acute infarction, low voltage may be an indicator of gobal hypocontractility from myocardial "stunning" (More on this topic in My Comment at the bottom of the page in the November 12, 2020 post of Dr. Smith's ECG Blog).
- Considering this overall reduced voltage — the T waves in high-lateral leads I and aVL of ECG #1 tower over the respective R waves in these leads (ie, these T waves are disproportionately tall).
- The shape of these high-lateral T waves is also abnormal — in being "hypervoluminous" (fatter-at-its-peak and wider-at-its-base than they should be).
- The diagnosis of OMI is confirmed by the location of reciprocal ST depression in all 3 inferior leads of ECG #1 — with a mirror-image picture in lead III (compared to aVL) and the terminal T wave positivity emphasized by Dr. McLaren.
- Finally — the shape of ST-T waves in leads V2-thru-V6 is clearly abnormal — which together with the RBBB pattern in lead V1, makes for 11/12 leads in this initial ECG that are clearly abnormal — in a 90-year old with unrelieved chest pain and worrisome bradycardia with her AFib. Therefore — Prompt cath is indicated.
- No matter how many times I see Brugada-1 and Brugada-2 ECG patterns — I still find myself referring to the images in Figure-2, that I reproduce from My Comment at the bottom of the pages of the April 17, 2022 and September 5, 2020 posts in Dr. Smith's Blog.
- What I found especially interesting about this "saddleback" pattern in lead V2 of ECG #1 — is that there is ST elevation in this lead — but not in either of its neighboring leads (ie, not in V1 or V3). When I first saw this tracing — I was not initially sure IF this ST elevation in lead V2 represented a pure Brugada-2 phenocopy (as a result of this patient's large acute MI) — OR — if part (or even all) of the ST elevation was a reflection of the "South African Flag" pattern from acute D-1 occlusion that Dr. McLaren refers to (See My Comment and illustration at the bottom of the page in the April 8, 2022 post of Dr. Smith's Blog for more on the S. African Flag pattern).
- The fact that the "saddleback" QRS morphology is not present in either the pre-hospital EMS ECG or in ECG #3 (shown above in Figure-1) — and — the fact that the "shape" of the upward concavity "saddleback" ST elevation in ECG #1 is not seen in either the EMS prehospital tracing or in ECG #3 (obtained ~2 hours after ECG #1) — to me suggests that there was both Brugada-2 phenocopy and evolving ST elevation from acute D-1 occlusion — and that as the OMI evolved, the Brugada-2 phenocopy pattern resolved!
Figure-2: Review of ECG Patterns in Brugada Syndrome (adapted from Brugada et al in JACC: Vol. 72; Issue 9; 2018) — (A) Brugada-1 ECG pattern, showing coved ST-segment elevation ≥2 mm in ≥1 right precordial lead, followed by a negative T-wave. (B) Brugada-2 ECG pattern (the “Saddle-back” pattern) — showing concave-up ST-segment elevation ≥0.5 mm (generally ≥2 mm) in ≥1 right precordial lead, followed by a positive T-wave. (C) Additional criteria for diagnosis of a Brugada-2 ECG pattern (TOP: the ß-angle; BOTTOM: A Brugada-2 pattern is present if 5 mm down from the maximum r’ rise point — the base of the triangle formed is ≥4).
- In addition to significant enlargement of the Q wave in lead V1 of ECG #3 — there is now ST elevation in this lead that was not present in ECG #1!
- That this is a real finding — is confirmed from new development of a Q wave in lead V2 and coved ( = "frowny"-configuration) ST elevation. Although the amount of this ST elevation in lead V2 is not great — its shape (ie, coved) is highly suggestive of acute evolving infarction — and the amount of ST elevation in V2 is clearly disproportionate compared to the tiny amplitude of the R wave in this lead.
- The location of this ST elevation in lead V2 + in leads I and aVL (with a comparable amount of reciprocal ST elevation in lead III) — fulfills lead location of the South African Flag pattern — which is diagnostic of the acute D-1 occlusion found on cath in today's case.
- When assessing ST-T wave changes for the likelihood of acute OMI — Shape, proportion and location are more important than the "amount" of ST elevation.






No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.