Written by Jesse McLaren, with comments by Smith and Grauer
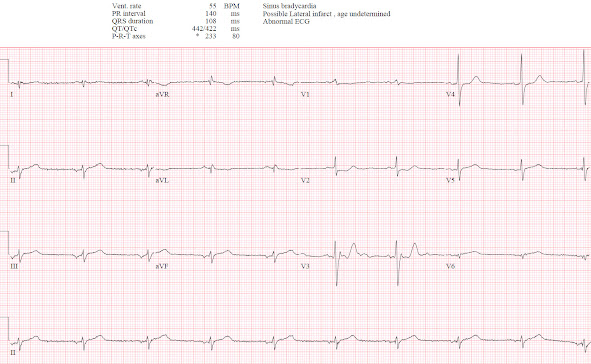
A 75 year-old presented with 8 hours of epigastric pain and one episode of vomiting. They had a history of gallstones but no cardiac history. Vitals were normal except a heart rate of 55, and below is the triage ECG. What do you think?
There’s bradycardia but it is not sinus: while the P wave is upright in I it is inverted in II so it is a low atrial rhythm. Conduction is otherwise normal, axis is indeterminate, and voltages are normal. There is early R wave progression with R>S in V1-2, primary ST depression in V2-3, and a Q wave with hyperacute T wave in V6. There is inferior ST elevation, but this could be secondary to atrial repolarization from the low atrial rhythm (see discussion below). But the inferior leads also have hyperacute T waves: broad, symmetric, and taller than the R wave. So this is a low atrial rhythm with infero-postero-lateral OMI.
Will you get posterior leads, and how will this change management?
Smith comment: this ECG is diagnostic of OMI; posterior leads can only be harmful by convincing you otherwise.
The emergency physician noted the anterior ST depression and was worried the patient was having ACS, but the ECG didn’t meet STEMI criteria. So, following STEMI guidelines, they gave aspirin and asked for a 15 lead ECG (below V5-6 are V8-9). This was done 20 minutes after the first ECG:
There is no ST elevation on the posterior leads, but there’s also less anterior ST depression. This confirms dynamic OMI but is still “STEMI negative.” So the patient had serial ECGs:
12 lead ECG done 50 minutes after the first ECG, patient still having pain:
Some normalization of anterior ST segment with slightly taller T wave in V3, and T waves inferiorly are smaller, suggesting some degree of reperfusion. But patient still having pain and ECG still STEMI(-)OMI.
110 minutes after the first ECG another 15 ECG lead was done, after the troponin I returned at 7,500 ng/L (normal <26 in males, <16 in females). The patient was still in pain, and now feeling presyncopal:
Now there is minimal posterior ST elevation but also more obvious anterior ST depression. The rhythm is now sinus with upright P waves in I/II, and without the positive repolarization wave there is still ST elevation and hyperacute T waves. The ECG is finally STEMI(+)OMI but the posterior leads were not needed: first, because there is more obvious changes anteriorly than posteriorly, and second because the patient is having refractory ischemia and a first troponin of 7,500 so they require immediate reperfusion regardless of the ECG.
Because the ECG finally met STEMI criteria the cath lab was activated: 100% proximal circumflex occlusion, with a peak troponin of 50,000 (a very large MI).
Below is the
discharge ECG two days later: back to low atrial rhythm, with inferior Q waves and T wave
inversion confirming the initial T waves were hyperacute, Q waves and
reperfusion T wave inversion laterally, and their equivalent posteriorly (anterior tall R waves and T waves):
By following the STEMI paradigm, ECG-to-Activation time was 2 hours, and door to balloon time was 3 hours and 20 minutes despite the fact the physician was concerned about MI and recognized primary anterior ST depression. But since it didn't meet STEMI criteria they recorded serial ECGs to see if one of them would finally met criteria. If the posterior leads hadn’t achieved minimal elevation, the patient probably would have been admitted as “NSTEMI” and had a much longer delay to reperfusion.
By following the OMI paradigm, this reperfusion delay could have been avoided. As Smith and Meyers found in the recent study on posterior OMI, ischemic ST depression maximal V1-V4 was 97% specific for OMI. Furthermore, "Among the 99 patients with OMI with STDmaxV1–4 (all of whom are presumed to benefit from emergent reperfusion), the 52 (53%) patients lacking STEMI criteria had similarly high peak troponin levels but significantly lower chance of receiving catheterization within 90 minutes of presentation. We believe that these patients in need of emergent reperfusion likely did not receive it because of the absence of STEMI criteria, despite the fact that they could have been identified easily and immediately by STDmaxV1–4, in addition to other subtle signs of OMI. Furthermore, among one-third of those with STEMI criteria, those criteria emerged a median of 1 hour after appearance of STDmaxV1–4; earlier diagnosis by STDmaxV1–4 could have resulted in earlier reperfusion."[1]
Emery phenomenon vs Occlusion MI
Atrial repolarization is in the opposite direction as depolarization, so when P waves are upright the atrial repolarization will be negative. This is usually small and buried in the QRS, but can sometimes continue beyond the QRS and simulate ST depression (see this case). But if P waves are inverted, atrial repolarization will be positive—and if it continues beyond the QRS complex can simulate ST elevation.
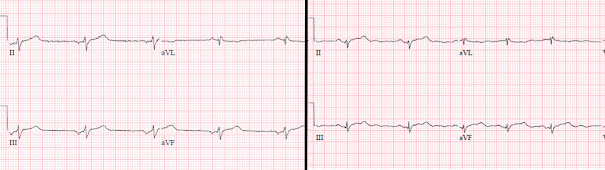
See this case and discussion of a patient who presented with chest pain, who appeared to have inferior ST elevation. On the left: large inverted inferior P wave, a positive and proportional atrial repolarization wave producing ST elevation (and reciprocal ST depression), and normal T wave (small, narrow point and asymmetric). On the right: repeat ECG after return of sinus rhythm, revealing no ischemic ST elevation and normal T waves:
In contrast, here are the ECGs from the case presented above. On the the left: low atrial rhythm with small P wave, disproportionate ST elevation and hyperacute T waves (relatively large, rounded peak and symmetric). On the right: return of sinus rhythm with ongoing ST elevation and hyperacute T waves:
Take home
1. low atrial rhythm can produce atrial repolarization that simulates ST elevation, but this should not produce disproportionate ST elevation or hyperacute T wave
2. primary anterior ST depression is specific for Occlusion MI without the need for posterior leads
3. STEMI criteria, including posterior leads, can be falsely negative and lead to delayed reperfusion
References:
1. Meyers HP, Bracey A, Lee D, et al. Ischemic ST-segment depression maximal in V1-V4 (versus V5-V6) of any amplitude is specific for occlusion myocardial infarction (versus nonocclusive ischemia). J of Amer Heart Assoc 2021
===================================
MY Comment by KEN GRAUER, MD (5/3/2020):
===================================
- I focus my comments on a number of fine points in addition to those highlighted by Dr. McLaren. To do this — I've reproduced the 1st and 4th tracings done in today's case (Figure-1).
-USE%20copy.png) |
| Figure-1: The 1st and 4th ECGs shown in today's case (See text). |
- The ED physician was appropriately concerned about anterior lead ST depression in ECG #1 — albeit millimeter-dependent STEMI criteria were not met.
- In addition, Dr. McLaren noted the ST elevation with hyperacute T waves in inferior leads — the finding of an R>S in leads V1,V2 — and, the Q wave with hyperacute T wave in lead V6.
- There is distinct low voltage (ie, None of the limb leads exceed 5 mm). QRS amplitude in 4/6 chest leads is also remarkably reduced (especially in leads V1 and V6). While low voltage is clearly a nonspecific ECG finding with a long differential diagnosis (which I reviewed in My Comment at the bottom of the page in the November 12, 2020 post of Dr. Smith's Blog) — there are several considerations relevant to today's case. Low Voltage may be the result of: i) Large body habitus (See below); and, ii) a large MI (from myocardial "stunning").
- Lack of an upright P wave in lead II of ECG #1 complicates assessment of the inferior lead ST-T wave changes. It's simply impossible to discount a potential effect by the Emery Phenomenon described above by Dr. McLaren. That said — in addition to small size of the negative P waves noted by Dr. McLaren in ECG #1 (ie, the size of the Ta [atrial repolarization] wave is proportional to the size of its P wave) — the PR interval for the negative P wave is not overly short, therefore far less likely to displace the oppositely-directed Ta wave as far rightward as I'd expect would be needed to influence the disproportionately large inferior lead T waves (See My Comment in the June 3, 2020 post).
- Also complicating assessment of ECG #1 — is the very unusual appearance of the QRS complex in several of the leads. I cannot recall the last time I saw such a tiny triphasic, yet predominantly negative QRS complex as we see in lead I. I initially thought that this had to represent some type of lead misplacement — but subsequent ECGs confirmed this highly unusual QRS morphology for lead I (See ECG #4 in Figure-1).
- I thought the appearance of lead V6 was also highly unusual. I completely agree with Dr. McLaren that the Q wave and clearly disproportionately large T wave in this lead strongly suggests acute lateral OMI. But I found it bizarre to move from the equiphasic R=S morphology with a 4.5 mm tall R wave in lead V5 — to a tiny, virtually all negative QRS complex in lead V6.
- Lead V3 is marred by artifact. In addition to the extra baseline deflections — the pseudo-r' at the end of each QRS makes no sense given how narrow the QRS complex is in 10/12 leads. Lead V1 is the other lead with pseudo-QRS-widening, as the angled initial part of the tiny QRS in that lead makes no sense.
- The "theme" of low voltage (in both limb and chest leads) — plus — the bizarre QRS appearance of lead I — the overly tiny (and unexpectedly all negative) QRS appearance of lead V6 — the pseudo-QRS-widening in lead V1 — and — the QRS and ST-T wave artifact rendering lead V3 as unreliable for ST-T wave assessment — in this patient being considered for possible acute coronary artery occlusion — should immediately prompt repeating the ECG. Support for the diagnostic validity of lead V2 could clearly be improved by availability of a valid recording that includes neighboring leads V1 and V3. I would not have waited to repeat the ECG.
- Possible explanations for the overly low voltage and unusual QRS morphology we see in several leads includes lead placement errors and body habitus. Is the patient of overly large body size?
- IF the patient was female — then I'd be wondering if the abrupt drop-off in R wave amplitude in lead V6 might be related to lateral chest lead electrodes being placed on top of (rather than under) breast tissue (Nice review in GE Healthcare-2022).
- The Mirror Test — is based on the premise that the anterior leads provide a mirror image of electrical activity in the posterior wall. By simply inverting a standard 12-lead ECG, and then holding it up to the light — you can easily visualize the "mirror-image" of leads V1,V2,V3.
- The advantage of the Mirror Test over the use of posterior leads — is that electrical activity does not have to traverse the thick back musculature — so ST segment deviations tend to be much easier to appreciate.
- To facilitate recognition of the clinical significance of the ST-T wave depression in lead V2 of ECG #1 — I've placed the mirror-image of this lead to the right of this tracing. Keep in mind that the mirror-image of this anterior lead gives us a picture of the electrical activity occurring in the posterior wall of the LV.
- ISN'T the mirror-image view of this anterior lead in ECG #1 all but screaming out, "I'm having an acute posterior OMI" (ie, large Q wave with hyperacute ST elevation and beginning T wave inversion)?
ECG #4 in Figure-1 was obtained 110 minutes after ECG #1. A sinus mechanism was restored (the P wave is now upright in lead II) — with sinus arrhythmia and at least 1 PAC as the rhythm. I thought this tracing insightful in showing comparison between use of the Mirror Test on the QRS complex in lead V3 — with the appearance of posterior leads V8 and V9 that were obtained at the same moment in time.
- Isn't it easier to appreciate the acute posterior OMI from the mirror-image of lead V3? — than from the smaller amplitude QRST complexes seen in the posterior leads?






No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.