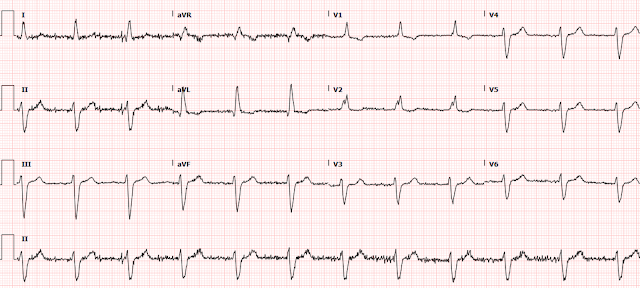
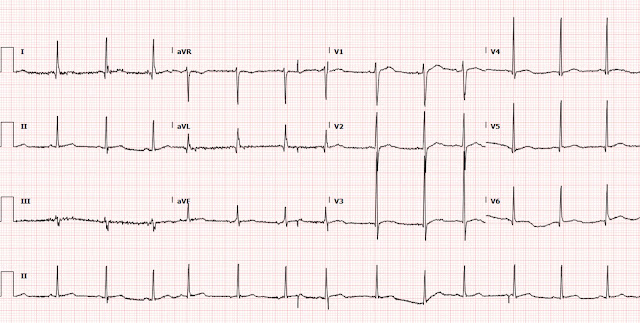
A 70 yo F with no previous cardiac history, but with a h/o hypertension, hyperlipidemia, and strong family history of ACS, presented with one hour of classic chest pain and appeared uncomfortable.
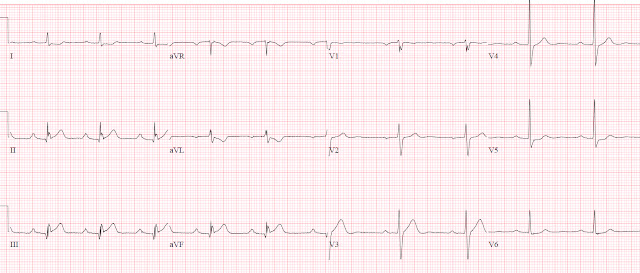
Her systolic BP was 210. The ECG is here:
A bedside echo was normal to that provider's eye (no bubble contrast).
She had a CT for dissection that was negative.
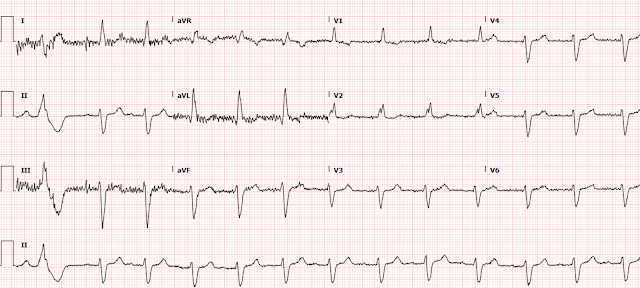
A 2nd ECG was recorded:
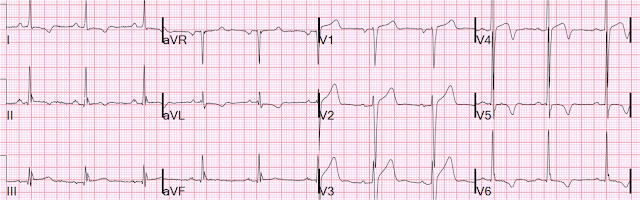
Here are the 2 ECGs (leads II, III, aVF, aVL) side by side:
First ECG 2nd ECG
The first time I looked at these was on my phone, and I did not notice the change. See how the J point in lead III has risen in the 2nd ECG (and become more reciprocally depressed in aVL). On the first, the ST/S ratio is 2/12 = 0.167 (16.7%); on the 2nd it is 2.5/11.5 = 0.217 (21.7%). Any value over 15% is suspicious, and a value over 20% still has 94% specificity for OMI in our validation study. See Ken Grauer's additional points below.
Moreover, when there is a change, with increase in ratio, it is much more specific.
Also, remember that New LBBB is no more likely than old LBBB in MI, and that only about 7% of patients with chest pain and LBBB in the ED have acute MI, with only about 3% having OMI.
See this review paper that I and others wrote in Current Cardiology Reports (we recommend use of the Cai and Sgarbossa algorithm which uses the Smith Modified Sgarbossa ECG Criteria): Diagnosis of Occlusion Myocardial Infarction in Patients with Left Bundle Branch Block and Paced Rhythms
Clinical Course:
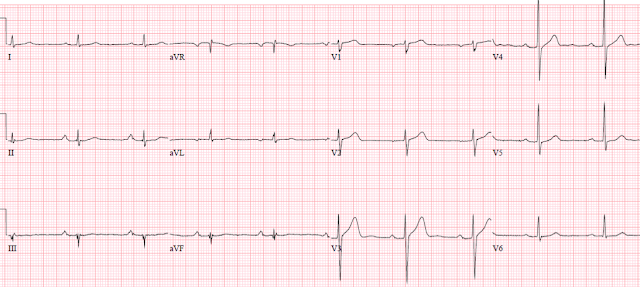
The physician tried to activate the cath lab, but that was unsuccessful. She was started on a nitroglycerin and heparin drip, and given full dose Aspirin. He was able to lower the BP to 140s systolic, and she became chest pain free. Cardiology did not want to cath her because she was chest pain free after nitro improved her blood pressure. He repeated the ECG again and it looked identical to the first one.
So the ischemia resolved, by both symptoms and ECG, with nitroglycerin and BP lowering.
The initial hs troponin I returned at 117 ng/L, then rose to 274 ng/L, then 1064 ng/L, then 1681 ng/L.
An echocardiogram was normal with 65% EF.
Next day angiogram showed: Severe diffuse atherosclerosis - 50-70% stenosis of LAD, prox ramus 70%, OM1 50%, RCA 20-30%. I do not know whether a culprit was found. She underwent 3x CABG (LIMA-pLAD, SVG-OM4, SVG-ramus). TIMI flow is not given, but probably was TIMI-3 flow (good flow) in all arteries.
Comment:
Our usual criteria for OMI is either an acute culprit lesion with TIMI 0-1-2 flow, or TIMI 3 with culprit and a peak hs troponin I of at least 10.0 ng/mL (equivalent to hs trop of 5000-10,000 ng/L) or troponin T of 1.0 ng/mL. So this case does not meet our strict criteria for OMI. Just having an intervenable culprit or lesion is not enough: there must also be <TIMI 3 flow, or a very high troponin.
Wall motion: if there is very brief occlusion, wall motion can quickly recover. Absence of a wall motion abnormality does NOT rule out OMI.
----In our study of 808 patients with suspicion for ACS, as the criteria were loosened (peak troponin level lowered), our sensitivity of the ECG for OMI diminished, and specificity increased; as criteria were more stringent (higher peak troponin required), sensitivity increased and specificity decreased). See Figure 3 for examples.
Nevertheless, it is likely that the artery was occluded, or nearly so, at the time of the 2nd ECG, and then reperfused by the time of the 3rd ECG, which reverted to the non-ischemic 1st LBBB tracing.
===================================
MY Comment, by KEN GRAUER, MD (1/31/2022):
===================================
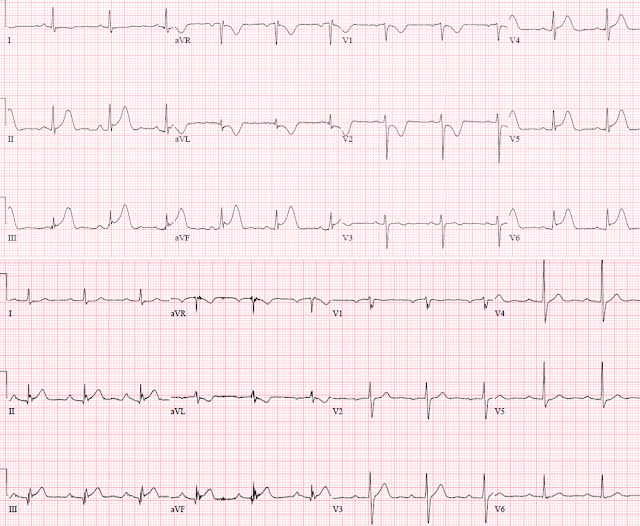
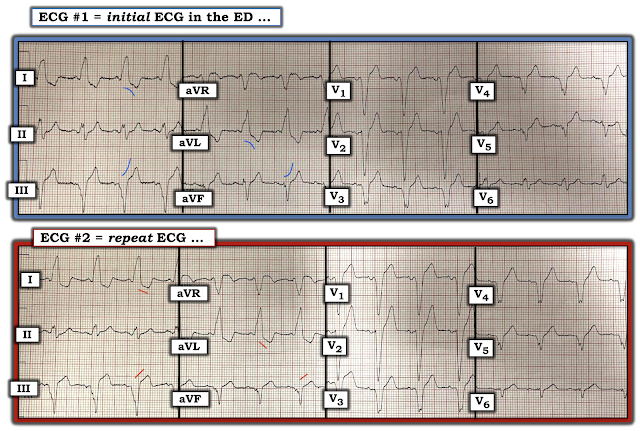
Important case by Dr. Smith for illustrating subtle but important differences in the 2 serial LBBB ECGs in this case. For clarity — I have placed the complete 12 leads of both tracings next to each other (Figure-1).
- Dr. Smith has already drawn attention to the increase in the amount of J-point ST elevation in lead III of ECG #2.
- As an additional feature to consider — I submit that ST-T wave morphology is clearly different between these 2 tracings — which immediately told me in this patient with typical chest pain that an acute event was in progress.
- The KEY to comparison of serial tracings is to: i) Interpret the initial tracing first in its entirety; and then, ii) Go lead-to-lead in your comparison of each of the 12 leads.
- Be SURE to check out QRS morphology in the 2 tracings you are comparing — since IF there has been an axis shift or difference in chest lead placement — this will need to be accounted for.
- QRS morphology in all 6 limb leads of ECGs #1 and #2 is quite similar! There is a slight difference in QRS morphology in lead V6 of ECG #2 — in that the QRS remains predominantly negative in lead V6 (whereas it was slightly more positive than negative in ECG #1). That said — overall QRS morphology is quite similar, which means that lead-to-lead comparison is valid!
- Note in ECG #1 that smooth shape of ST-T waves in virtually all limb leads (slightly curved BLUE lines in ECG #1).
- In contrast — note straightening of the ST segments in these same leads in ECG #2. Together with the increase in J-point ST elevation already noted by Dr. Smith — there is no doubt that at least 4 of the 6 limb leads in ECG #2 (short RED lines) are different. In a patient who clearly has underlying heart disease (virtually all patients with LBBB do!) + new onset "classic" chest pain — these ST-T wave morphology changes merit prompt cath!
 |
| Figure-1: Comparison of both 12-lead ECGs in today's case. |