A 60-something woman with no cardiac history presented with epigastric and right upper quadrant pain after eating spicy food.
She had an ECG recorded at triage:
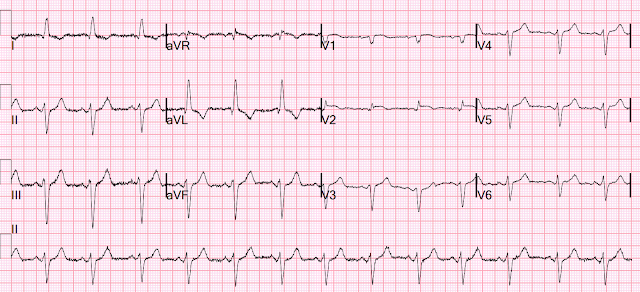
This was sent to me without any info while I was out and about, and I looked at it on my phone. I responded: "That is a tough one. V2 is very worrisome. But no other leads are. I probably would call it OMI. What was the outcome?"
More analysis while looking at it on a computer screen: There is an intraventricular conduction delay with appropriately discordant ST segments and T-waves. But there is also concordant STE in V2. The STE in V1 is out of proportion to the S-wave, so V1 is also very worrisome (something I did not see on my phone).
It turns out that the conventional algorithm was also worried, and because of that, the patient was brought to the critical care area.
Here is that interpretation of the conventional computer algorithm:
SINUS RHYTHM
INTRAVENTRICULAR CONDUCTION DELAY [130+ ms QRS DURATION]
LEFT VENTRICULAR HYPERTROPHY AND ST-T CHANGE [VOLTAGE CRITERIA PLUS ST/T ABNORMALITY]
POSSIBLE SEPTAL MYOCARDIAL INFARCTION , PROBABLY RECENT [30 ms Q WAVE IN V1/V2]
LATERAL MYOCARDIAL INFARCTION , PROBABLY RECENT [40+ ms Q WAVE AND/OR ST/T ABNORMALITY IN I/aVL/V5/V6]
***ACUTE MI***
Later, there was an overread by a Cardiologist, who removed the ***Acute MI***
SINUS RHYTHM. No Previous ECGs Available.
INTRAVENTRICULAR CONDUCTION DELAY [130+ ms QRS DURATION]
LEFT VENTRICULAR HYPERTROPHY AND ST-T CHANGE
ABN R PROGRESSION, ASMI OR LEAD PLACEMENT
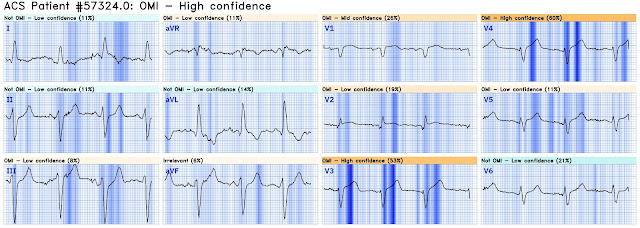
The providers ran the ECG through the Queen of Hearts:
Like good physicians, they recorded another ECG at 10 min:
Now there is S-wave shortening in multiple precordial leads, rendering the T-waves hyperacute! The T-waves have barely changed, but the S-waves have shortened and thus the T/QRS ratio is much larger (that is both T-wave amplitude and T-wave area under the curve).
Now it is diagnostic of OMI.
Here is the conventional algorithm:
SINUS RHYTHM
INTRAVENTRICULAR CONDUCTION DELAY [130+ ms QRS DURATION]
ABNORMAL ECG
And the Cardiology overread:
SINUS RHYTHM
INTRAVENTRICULAR CONDUCTION DELAY [130+ ms QRS DURATION]
ABN R PROGRESSION, ASMI OR LEAD PLACEMENT
Comparison Summary: NO SIGNIFICANT CHANGE.
Compared with: 8/18/2024 6:08 AM
This time neither see acute MI.
But the Queen of Hearts now sees it:
Click here to sign up for Queen of Hearts Access
Unfortunately, the team did not see it and they did not use the Queen on the 2nd ECG. So it was not until 68 minutes that they recorded another ECG:
The conventional algorithm diagnosed it:
SINUS RHYTHM
INTRAVENTRICULAR CONDUCTION DELAY [130+ ms QRS DURATION]
LEFT VENTRICULAR HYPERTROPHY AND ST-T CHANGE [VOLTAGE CRITERIA PLUS ST/T ABNORMALITY]
ANTEROLATERAL MYOCARDIAL INFARCTION , PROBABLY RECENT [40+ ms Q WAVE IN I/aVL/V3-V6]
***ACUTE MI***
And the Cardiology overread see the evolution but is not convinced:
SINUS RHYTHM
INTRAVENTRICULAR CONDUCTION DELAY [130+ ms QRS DURATION]
LEFT VENTRICULAR HYPERTROPHY AND ST-T CHANGE [VOLTAGE CRITERIA PLUS ST/T ABNORMALITY]
ANTERIOR AND LATERAL ST CHANGES
ABN R PROGRESSION, ASMI OR LEAD PLACEMENT.
Comparison Summary: ANTEROLATERAL ST CHANGES ARE NOW MORE PRONOUNCED, RATE DEPENDENT VS ISCHEMIC CHANGES
Here is the Queen's diagnosis:
The cath lab was activated:
Culprit Lesion (s): Thrombotic 99% mid LAD stenosis with TIMI II flow
Peak troponin not measured, unfortunately. So we don't have a good idea how large the final infarct size was.
Echocardiography:
Normal left ventricular size with mild to moderately reduced LV systolic function; estimated LVEF is 44 %.
There is akinesis of the distal septum, anterior, apex, and distal inferior wall consistent with LAD territory ischemia or infarction.
How large is the infarct?
It is impossible to conclude from this that the infarct was VERY large, though it likely was very large since the time to intervention was long. The area of insult was indeed large, but whether that area is all irreversibly infarcted or not would require MRI or a 6 week followup ("convalescent") echocardiogram, to see how much myocardium recovers (was only "stunned", not infarcted)
Post PCI EKG:
Learning Points:
Serial ECGs are critical.
Apply the Queen of Hearts to all serial ECGs.
Occasionally, the conventional algorithm will beat the Queen
Cardiology overreads are not sensitive for OMI.
MY Comment, by KEN GRAUER, MD (8/26/2024):
- Although clearly a history of abdominal pain suggesting cholecystitis — an ECG was appropriately done. This is an important reminder of the many ways other than chest pain, that acute coronary syndromes may present (See Talakic et al — Cardiovasc Med, 2023 and Tsipouras — Austral Fam Phys 37(8), 2008, among many other sources).
- As per Dr. Smith — there is no way that the QRST complex in lead V2 can be normal (within the RED rectangle in Figure-1). There is abnormal ST segment coving — and — considering how small QRS amplitude is in this lead, there is marked ST elevation.
- There is also a fragmented and a markedly widened initial Q wave in lead V2.
- Finally — there is an "orphan" (ie, predominantly tall) R wave in lead V2 — which is distinctly different from all other chest leads (See below).
- The limb leads in ECG #1 show sinus rhythm with QRS widening and marked LAD (Left Axis Deviation). Although the frontal plane axis of -60 degrees is negative enough to be consistent with LAHB (Left Anterior HemiBlock) — with "pure" LAHB, the initial r waves in the inferior leads are generally not as wide as seen here — so a "nonspecific" conduction delay with marked LAD might be a better description of findings. That said — ST-T wave findings in the limb leads are non-diagnostic! Both LVH (which may be present here) and LAHB may be associated with the ST-T depression seen here in leads I and aVL.
- BUT — Other Chest Leads in ECG #1 are not normal — especially the neighboring leads to lead V2. As per Dr. Smith — the relative amount of ST elevation in lead V1 is disproportionate to the tiny S wave depth in this lead (BLUE arrow in lead V1).
- As we often emphasize — there normally will often be slight, gently upsloping ST elevation in lead V3 — but no J-point elevation is seen here in this lead (BLUE arrow in V3). The reason that I found this subtle finding in lead V3 important — is the subtle-but-real "extra" amount of ST elevation that is present here in lead V4, which makes for concerning findings in the first 4 chest leads. I would not normally expect this much ST elevation in lead V4 given the lack of any ST elevation in lead V3.
- In Summary: I was not certain about there being an acute OMI from my initial assessment of ECG #1. That said — I thought the chest lead findings in leads V1-thru-V4 suspicious enough that I was glad a repeat ECG was obtained within the next 10 minutes!
- #1 — There is no longer any question about acute LAD OMI! Today's case provides yet another example of how quickly definitive ECG changes can evolve — with directive to us, that we have a low threshold for repeating the ECG when concerned about a less-than-diagnostic initial ECG within the next 10-to-20 minutes. Continue repeating serial ECGs until such time that definitive management plans are made (sometimes meaning until you can convince the on-call interventionist to take the patient to the cath lab!).
- #2 — The BEST way to get good at detecting early acute signs of OMI — is to routinely GO BACK to the initial tracing after ST-T wave changes have evolved. Doing so in Figure-1 facilitates recognizing how the subtle abnormalities I highlighted above in leads V1, V3 and V4 have evolved to obvious diagnostic findings.
- While possible that electrode lead placement of lead V2 in today's case is correct — given that the most remarkably abnormal lead in today's initial ECG is lead V2 (as the only chest lead with a predominant R wave) — ensuring accurate electrode lead placement would seem essential for accurate diagnosis. BEST practice when suspecting potential lead misplacement of a critical lead in your assessment — is to verify lead placement, and immediately repeat the ECG.
- The mechanism proposed for the isolated finding of a tall R wave in lead V2 in a patient with acute chest pain is complex and not fully elucidated. It is thought to result from abnormal depolarization of an acutely ischemic area that leads to dispersion of repolarization times — such that unopposed positive forces facing an area of acute ischemia produce the isolated tall R wave in lead V2 (ie, repolarization dispersion during acute ischemia may lead to transient obliteration of the S wave — therefore leaving an unopposed R wave).
- In the case study by Chugh et al — the tall R in V2 was transient, resolving after acute ischemia was controlled. In today's case — the tall R in V2 resolved after successful PCI.
- Editorial Note: I have seen this ECG finding before — but did not previously realize that rather than lead misplacent, this might be an indication of acute OMI. I will as of now be "on the lookout" for more examples, that I'll be correlating with clinical events to see if this finding portends acute LAD occlusion.
-USE%20copy.png) |
| Figure-1: Comparison between the first 2 ECGs in today's case. |









.jpg)




-USE.png)