Written by Colin Jenkins and Nhu-Nguyen Le with edits by Willy Frick and by Smith
A 46-year-old male presented to the emergency department with 2 days of heavy substernal chest pain and nausea. He reported a history of “Wolf-Parkinson-White” and “heart attack” but said neither had been treated. These diagnoses were not found in his medical records nor even a baseline ECG. He had no previously documented medical problems except polysubstance use. An ECG was obtained shortly after arrival:
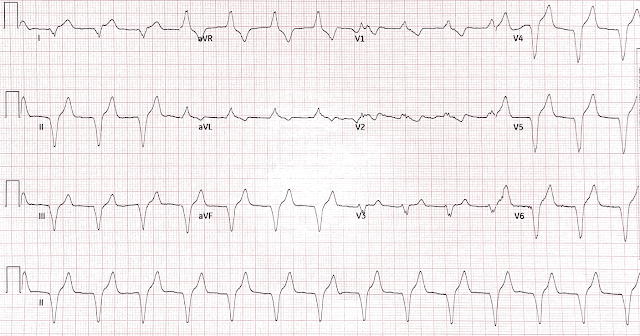
- STE and HATW across the precordium
- Q waves in V1-V4
- Subtle STE and HATW in I and aVL
- Terminal TWI suggestive of early reperfusion most pronounced in I, aVL, V5, and V6
- Reciprocal STD with ischemic down-up T waves in II, III, aVF
- Both patients and other medical providers can report confusing and often contradictory information that obfuscates the diagnosis (in this case, WPW). If the story and ECG findings are concerning, use your clinical judgment to advocate for PCI.
- Perform serial ECGs even if symptoms are constant. Serial ECGs enhance the diagnosis of acute coronary syndrome. However, Smith and Meyers have proven that so called "early" findings such as hyperacute T-waves often do not evolve to diagnostic ST Elevation.
- AIVR is not always the result of significant pathology, but is classically associated with the reperfusion phase of acute myocardial infarction.
- Similarly, the OMI paradigm respects ACS as a dynamic process in which ECG changes reflect the phase of myocardial injury and risk stratify which patients may benefit from emergent PCI.
- Although recognition of OMI was not affected by administration of morphine in this case, use caution with analgesia in ongoing ACS without a definitive plan for angiography. Opioids associate with worse outcomes in myocardial infarction, probably because they eliminate the pain signal that informs the clinician of the urgency of revascularization.
- Do not treat AIVR. In fact, use of antidyrhythimcs (e.g., lidocaine) can result in severe bradycardia or asystole (Weinberg, Sedowski and Alexander, below)
- The presence of accelerated idioventricular rhythm does not affect prognosis, and there is no definitive evidence that, if left untreated, the incidence of VF or death is increased. In other words, it is a safe rhythm. Leave it alone. (Bigger et al. below)
Ferrier, G. R., Moffat, M. P., & Lukas, A. (1985). Possible mechanisms of ventricular arrhythmias elicited by ischemia followed by reperfusion. studies on isolated canine ventricular tissues. Circulation Research, 56(2), 184–194. https://doi.org/10.1161/01.res.56.2.184
Fesmire, F. M., Percy, R. F., Bardoner, J. B., Wharton, D. R., & Calhoun, F. B. (1998). Usefulness of automated serial 12-lead ECG monitoring during the initial emergency department evaluation of patients with chest pain. Annals of Emergency Medicine, 31(1), 3–11. https://doi.org/10.1016/s0196-0644(98)70274-4
Kaplinsky, E., Ogawa, S., Michelson, E. L., & Dreifus, L. S. (1981). Instantaneous and delayed ventricular arrhythmias after reperfusion of acutely ischemic myocardium: Evidence for multiple mechanisms. Circulation, 63(2), 333–340. https://doi.org/10.1161/01.cir.63.2.333
Meine, T. J., Roe, M. T., Chen, A. Y., Patel, M. R., Washam, J. B., Ohman, E. M., Peacock, W. F., Pollack, C. V., Gibler, W. B., & Peterson, E. D. (2005). Association of intravenous morphine use and outcomes in acute coronary syndromes: Results from the Crusade Quality Improvement Initiative. American Heart Journal, 149(6), 1043–1049. https://doi.org/10.1016/j.ahj.2005.02.010
Other References
Weinberg B, Zipes D. Strategies to manage the post-MI patient with ventricular arrhythmias. Clin Cardiol 1989;12:86–90.
Sadowski ZP, Alexander JH, Skrabucha B, et al. Multicenter randomized trial and a systematic overview of lidocaine in acute myocardial infarction. Am Heart J 1999;137:792–8
Alexander JH, Granger CB, Sadowski Z, et al, for the GUSTO-I and GUSTO-IIb Investigators. Prophylactic lidocaine use in acute myocardial infarction: incidence and outcomes from two international trials. Am Heart J 1999;137:799–805.
Bigger JR Jr, Dresdale RJ, Heissenbuttel RH, et al. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis1977;19:255–300.
MY Comment, by KEN GRAUER, MD (8/9/2024):
- Ironically — it is fortunate that this patient had continued CP (Chest Pain) and an Echo showing a localized wall motion abnormality — as those 2 factors apparently were the reason that cardiac cath was performed.
- For clarity in Figure-1 — I've reproduced and labeled the 3rd ECG shown by Dr. Frick (which was the 2nd of the 3 repeat ECGs obtained over a 5-minute span).
- To "set the scene" for ECG #3 (that I show in Figure-1) — the patient had ongoing CP at the time this ECG was done — with the diagnoses of "No STEMI" and unstable agina being made for this and the other serial tracings despite return of a markedly increased troponin level.
- Interpretation of the acute findings in ECG #3 is best accomplished by first understanding the rhythm. I've labeled sinus P waves with RED arrows. Note the underlying sinus arrhythmia, with fairly marked variation in the P-P interval (with greatest slow-down between beats #6-to-7).
- Although P waves are partially hidden within portions of the QRS for the first 2 beats, as well as for the last 6 beats in this tracing — RED arrows over the QRS for each of these wide beats highlight "extra deflections" occurring at various points around the QRS, that clearly indicate partially hidden P waves that appear to be essentially "on time" considering the underlying sinus arrhythmia.
- Note that the QRS complex of beat #7 is a little smaller in the long lead II and long lead V5 rhythm strips compared to the QRS size of beats #8-thru-13. This is because beat #7 is a fusion beat (ie, the RED arrow P wave seen just before the QRS of beat #7 does not have enough time to be fully conducted to the ventricles). The presence of fusion beats proves that the wide beats in this tracing are all of ventricular etiology.
- The rate of these wide beats in ECG #3 is ~75/minute. As per Dr. Frick — this defines the ventricular rhythm as AIVR (Accelerated IdioVentricular Rhythm).
- The reason AIVR is seen in ECG #3 — is that the rate of the underlying sinus arrhythmia slows after beat #5 — and once the sinus rate drops below the accelerated ventricular escape rate, AIVR takes over (Laddergram illustration of the mechanism of this rhythm is shown below in Figure-2).
- Although tiny in size — the BLUE arrows highlight definite ST elevation in leads I and aVL. ST segments in both of these leads are abnormally straightened — and there is terminal T wave inversion (which is more obvious in lead aVL). Considering the tiny QRS — the Q wave in lead aVL is huge.
- Each of the sinus-conducted beats in the 3 inferior leads show reciprocal ST depression.
- In addition — the ventricular beats in leads I,II,III show disproportionately "bulky" T waves and inappropriate ST segment deviation (ST elevation for beats #1,2 in lead I — and ST depression for beats #1,2 in leads II,III).
- While more difficult to assess the "appropriateness" of J-point elevation in the chest leads because of the AIVR rhythm — lead V6 clearly shows disproportionate J-point ST elevation, consistent with acute OMI.
- As per Dr. Frick — in the setting of an ongoing acute OMI, the occurrence of AIVR is often a favorable sign indicative of at least some degree of reperfusion. No treatment of AIVR is needed if the patient is hemodynamically stable — since this rhythm often serves as an appropriate "escape" rhythm due to transient slowing of the sinus pacemaker (ie, which is exactly what happens beginning with beat #7 in ECG #3).
- CAVEAT: The "atrial kick" is lost with AIVR (since P waves are no longer conducting once AIVR takes over). As a result — cardiac output may sometimes decrease enough to produce symptomatic hypotension. When this occurs — a low dose of Atropine is the treatment of choice — as this will usually speed up the SA node enough for sinus rhythm to take over (with resumption of effective atrial contraction). That said, most of the time — AIVR is a short-lived and benign rhythm (as well as an encouraging sign that at least some reperfusion is occurring).
-labeled-USE.png) |
| Figure-1: The initial ECG in today's case. (To improve visualization — I've digitized the original ECG using PMcardio). |
-USE.png) |
| Figure-2: Laddergram illustration showing takeover over AIVR, beginning with fusion beat #7 — as a result of slowing of the rate of the underlying sinus arrhythmia. |














No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.