Written by Colin Jenkins. Colin is an emergency medicine resident beginning his critical care fellowship in the summer with a strong interest in the role of ECG in critical care and OMI.
Edits by Willy Frick.
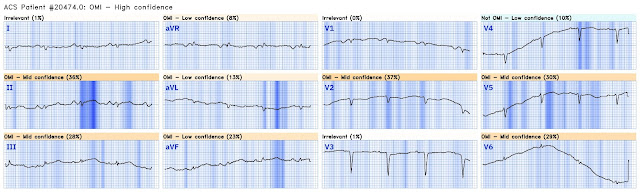
ECG 1
What do you think?
The ECG has a lot of artifact, and the amplitude is very small, making interpretation challenging. We can see enough to make out that the rhythm is sinus tachycardia. Tachycardia is unusual for OMI, unless the patient is in cardiogenic shock (or getting close). The lack of anterior R waves suggests age indeterminate anterior MI, but there does not appear to be active anterior OMI.
Sometimes when the amplitude is this small and the signal to noise ratio is this poor, it can be difficult to be sure what is the QRS, the ST segment, the T wave, etc. It is often helpful to find a lead where the distinction is obvious, for example the V1 rhythm strip. You can then compare them to help clarify. This is shown below with lead V6. The vertical lines mark the beginning and end of the QRS (clearly identifiably on the rhythm strip and carried upward), and the horizontal line marks the isoelectric baseline.
By itself, V6 could be overlooked. When you compare it to V1, you can immediately appreciate the huge area under the curve in the V6 T wave, strongly suggestive of hyperacute T wave. By inspecting neighboring territories, aVF suddenly becomes much more suspicious, and to a lesser extent lead II. Altogether, this strongly suggests inferolateral OMI, particularly in a patient with acute chest pain.
Remember that ECG machines generally have modifiable gain. Standard gain is 10 mm/mV, but if you have a low voltage tracing like this, you can repeat it with gain increased to 20 mm/mV. Here is ECG 1 with gain increased to 20 mm/mV.
Now it is a little bit easier to appreciate the inferolateral ST elevations, although there is still a lot of artifact. I sent the original gain ECG to Dr. Meyers with no clinical context, and he said "OMI."
Here is Queen of Hearts explainability:
As you can see, the Queen has overall Mid Confidence of OMI, but high confidence for lead V6
YOU TOO CAN HAVE THE PM Cardio AI BOT!! (THE PM CARDIO OMI AI APP)
If you want this bot to help you make the early diagnosis of OMI and save your patient and his/her myocardium, you can sign up to get an early beta version of the bot here.
I also texted the ECG to Dr. Smith. His response was: "OMI. V6."
Due to artifact, a second ECG was obtained.
ECG 2
Especially in the context of the first ECG, readers of this blog will readily appreciate the ST elevations and hyperacute T waves in II, III, aVF, V6, and to a lesser extent V5. And compared to ECG 1, we now appreciate downsloping ST depression in V1 and V2 consistent with posterior extension. (Remember that the posterior wall is in between the inferior and lateral walls, so this is not surprising.)
Here is the Queen's interpretation, with Explainability:
The clinicians taking care of the patient did appreciate the inferior STE, but noted that it was less than 1 mm.
Smith comment: this is diagnostic of OMI until proven otherwise. A bedside ultrasound should be done to assess volume and other etiologies of tachycardia, but if no cause of type 2 MI is found, the cath lab should be activated NOW. Do not wait for the troponin; a lot of myocardium will be dead if you do. The "flu-like" illness suggests myo- or pericarditis, but that would be a diagnosis of exclusion.
The case continues...
Some time later, high sensitivity troponin I drawn at the time of ECG 1 resulted at 25,400 ng/L (ref. 3-54). This confirmed the clinicians' suspicion for subtle OMI, prompting activation of the cath lab. A third ECG was obtained which is shown below:
ECG 3
Here is Queen of Hearts explainability:
I sent the original gain ECG to Dr. Smith without any clinical context, and he answered “very subtle inferior posterior OMI”. The ECG more clearly demonstrates:
- Large area under the curve of the T waves in II, II, and aVF as well as V5 and V6 relative to the QRS complexes
- Straight, angulated ST segments in these same leads
- Depressed ST segments in V2-V3 (as would be expected in posterior OMI)
Cardiology took the patient for immediate angiography. While awaiting transfer to the cath lab, STAT echocardiogram was performed and showed LVEF 30-35%, as well as anterior, inferior, and apical hypokinesis, and apical thrombus. This confirms the suspicion of prior anterior OMI.
The thrombus is circled below in red. The orange arrow points to the anterolateral papillary muscle.
Shown below is the right anterior oblique caudal view ("RAO caudal"). This view is generally best for the left circumflex and its branches.
We see the LCx which gives off a few very small branches and then one larger OM. If you look closely at the top red arrow, you can see it points to a section with a small filling defect. This suggests thrombus (since the contrast does not penetrate the formed thrombus very well). In addition, the top left blue arrow indicates a section in the LAD with a severe stenosis, likely the culprit for the prior LAD occlusion which has since recanalized. There are also diagonal branches which are not well visualized.
Shown below is the left anterior oblique caudal view ("LAO caudal," or "spider shot"). This view is generally best for distal left main, proximal LAD and diagonal branches, and proximal LCx and branches.
We see the LAD coursing anteriorly and giving off diagonal branches. Again, the top blue arrow points to the likely prior site of LAD occlusion. We again appreciate the filling defect in the LCx at the top red arrow head. The OM courses inferolaterally.
Shown below is the left anterior oblique cranial view ("LAO crani"). This view is generally best for viewing the mid LAD along with diagonal bifurcations.
We again see the LAD coursing anteriorly. The diagonal branches overlap with the LCx and smaller OMs. The LCx thrombus at the red arrow is not well appreciated in this view because it overlaps with the LAD. We also see the larger OM in this view. In particular, this view showcases the very slow TIMI 2 flow in the LAD. This is due to prior infarct in this area and microvascular dysfunction.
Putting all the data together, the patient likely suffered an anterior OMI in the days or weeks prior to presentation. This resulted in anterior/apical infarct and apical thrombus formation. Then, part of the thrombus embolized into the LCx causing an inferoposterolateral OMI. (As an aside, the LCx OMI is a type 2 event, since it is due to supply-demand mismatch from thrombus, and not due to atherosclerotic plaque rupture or erosion).
Learning points:
- Repeat ECGs when artifact limits interpretation or diagnosis is uncertain.
- Increase the gain on your ECGs if the amplitude is very small.
- Use the rhythm strip to help identify the components of a noisy lead.
- As discussed on this blog many times before, proportionality is key to the diagnosis of OMI by ECG. Expecting to find 1 mm of STE on an ECG with diminutive amplitude is like expecting to find your house on a globe. The scale is wrong.
===================================
MY Comment, by KEN GRAUER, MD (1/15/2024):
===================================
I was not taught about artifact in either medical school or during my residency. Instead (as I'm sure many readers can relate to) — I learned about artifact from cases for which I missed the diagnosis. I was often in "good company" — because many clinicians far more experienced than I at the time, also failed to realize which ECG deflections were and were not "real".
Today's case by Drs. Colin Jenkins and Willy Frick offers a superb illustration of how despite overwhelming artifactual distortion — strong suspicion of acute OMI is still possible.
- In Figure-1, to reinforce KEY concepts put forth by Drs. Jenkins and Frick — I offer 3 additional examples of artifactual distortion (excerpted from my ECG Blog) — that resulted in arrhythmia misdiagnosis.
The CHALLENGE:
In each case — HOW can you immediately discount the arrhythmia that was diagnosed?
- Rhythm A: This rhythm strip was observed on telemetry — and thought to be AFlutter.
- Rhythm B: This patient was seen in the ED — and thought to be in AFlutter with 4:1 AV conduction.
- Rhythm C: This telemetry strip from an older adult was initially thought to need defibrillation.
-USE.png) |
| Figure 1 – Examples of artifactual distortion (excerpted from my ECG Blog) — that resulted in arrhythmia misdiagnosis. |
ANSWERS:
I've labeled in Figure-2 — KEY features for each of the 3 rhythm strips shown in Figure-1 that allow immediate recognition of the arrhythmia diagnosis:
- Rhythm A: We can immediately discount the possibility of AFlutter because: i) This rhythm lacks the consistent "sawtooth" pattern of typical AFlutter that should be seen in the inferior leads, in which each flutter wave looks the same; and, ii) The deflections in Rhythm A look geometric (ie, vertical) — they are irregular — and they occur at a much faster rate (ie, between 400-500/minute) compared to the 250-to-350/minute range expected for untreated AFlutter.
- A quick glance at this patient confirmed that these small amplitude vertical deflections in Rhythm A were the result of tremor artifact.
- Rhythm B: Next to 2:1 AV conduction — 4:1 AV conduction is common with AFlutter, which is what Rhythm B was initially thought to be. However, as was the case for Rhythm A — we can immediately discount AFlutter as the etiology of Rhythm B because: i) The small upright deflections on the baseline between QRS complexes are definitely not regular (as they should be with AFlutter); ii) There is a changing relationship between these small vertical deflections (that are seen throughout the baseline on this rhythm strip) and neighboring QRS complexes — whereas there is usually a repetitive pattern for the relationship between atrial deflections and neighboring QRS complexes with AFlutter; and, iii) We can make out the underlying rhythm (ie, RED arrows highlight upright sinus P waves!).
- Once again, a quick glance at the patient revealed a Parkinsonian tremor. The frequency of the characteristic resting tremor with Parkinson's disease typically approximates the rate of AFlutter.
- Rhythm C: As soon as Rhythm C was seen on telemetry — medical staff rushed into the patient's room. As you may have guessed — this patient was fine. ECG features in Rhythm C that allow us to immediately discount VFib include: i) The geometric appearance of these unphysiologic vertical deflections that are unpredictably irregular, and exceedingly rapid (well over 300/minute); and, ii) We can make out the underlying rhythm (Stepping back from the tracing suggests several deflections marked "X" that look like they might represent underlying QRS complexes. Setting our calipers to the interval between these complexes allows us to march out regularly-occurring deflections at a rate of ~130/minute thoughout the tracing = RED arrows).
LEARNING Points:
Artifact is much more common in clinical practice than many providers realize. At times, it may be extremely difficult to distinguish artifact from a real tracing. The consequences of overlooking artifact are substantial (ie, An acute OMI may be missed, as almost occurred in the case presented above by Drs. Jenkins and Frick — or additional procedures may be ordered and/or patients shocked for artifact simulating VT/VFib). Keep in mind the following:
- Check on patient first. The most likely causes of artifact include inconsistent skin-electrode contact and body movement (scratching, tremor, shivering, coughing, hiccups, brushing teeth, writhing in bed, seizure activity, interference from a mechanical device, etc.).
- ECG features that suggest artifact as the cause include geometric appearance (unphysiologic vertical deflections) that are unpredictably irregular, often at exceedingly rapid rates. On a 12-lead tracing, one often inexplicably sees highly unusual deflections in some leads, but not in others.
- Being able to identify an underlying regular rhythm that is undisturbed by artifactual deflections provides proof that the phenomenon is not real (as shown in Rhythms B and C in Figure-2).
-USE.png) |
| Figure 2 – KEY features for confirming artifact. |
================================
Links to Examples of ARTIFACT:
What follows below is an expanding list of technical "misadventures" — most from Dr. Smith's ECG Blog — some from other sources (NOTE: As I did not previously keep track of these — there are additional examples of artifact sprinkled through Dr. Smith's ECG Blog that I have not yet included here ... ).
- The September 15, 2023 post — for PTA (Pulse-Tap Artifact).
- The April 6, 2023 post — excessive baseline artifact misdiagnosed as AFib (instead of sinus rhythm with AV Wenckebach — as in Figure-4 in this post).
- The March 17, 2023 post — for PTA.
- The January 17, 2023 post — for PTA.
- The October 21, 2022 post — for "artifactual VT".
- The November 10, 2020 post — for PTA.
- The October 17, 2020 post — for a 70-year old woman with "Artifactual VT".
- The September 27, 2019 post — for the Rowlands & Moore article with the above-noted formulas for recognizing the “culprit” extremity.
- The September 22, 2019 post — intermittent ST-T wave artifact.
- The August 26, 2019 post — baseline artifact.
- The January 30, 2018 post — for PTA.
- Brief review by Tom Bouthillet on some common causes of artifact.
- Additional review of ECG artifacts by Pérez-Riera et al (Ann Noninvasic Electrocardiol 23:e12494, 2018)
- VT Artifact — by Knight et al: NEJM 341:1270-1274, 1999.
- Artifact simulating VFib — CLICK HERE.
- More VT-VFib artifact — CLICK HERE.
- Artifact simulating AFlutter — CLICK HERE.
- Parkinsonian Tremor vs AFlutter — CLICK HERE.
- Left Leg artifact — CLICK HERE.
- Should the cath lab be activated? — CLICK HERE.














No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.