This is written by Willy Frick, an amazing cardiology fellow in St. Louis.
https://twitter.com/willyhfrick
A 62 year old man with a history of hypertension, type 2 diabetes mellitus, and carotid artery stenosis called 911 at 9:30 in the morning with complaint of chest pain. He described it as "10/10" intensity, radiating across his chest from right to left. EMS obtained the following vital signs: pulse 50, respiratory rate 16, blood pressure 96/49. The ambulance report says "BP continued to drop during transport and pt remained cold and clammy." It appears EMS obtained two EKGs, but unfortunately these were not saved in the medical record. The EMS crew was only BLS certified, so EKG interpretation is not within their scope of practice.
The patient arrived just after 10 AM, and the following EKG was obtained. cTnI drawn at the same was 0.011 ng/mL (ref. <0.032 ng/mL). He was experiencing severe chest pain at the time.
EKG 1
There is obvious ST elevation most pronounced in V2 and V3. Despite this, the Queen of Hearts has only mid confidence that this is OMI. Why might that be? The QTc is 394 ms, which is on the short side for OMI. The T waves in V2 and V3 are very tall, but not particularly wide based as you would expect for classic hyperacute T waves. And the ST segments are very concave. The overall look of the tracing seems a little bit "off." I sent this EKG to Dr. Meyers and Dr. Smith with no other information. Dr. Meyers said "I think it's real. That TQRSD is worth a false positive in this situation." Dr. Smith said: "I would say it is fake EXCEPT that there is TQRSD in V2, V3. So one must take a look at the LAD."
This blog has extensively covered terminal QRS distortion AKA TQRSD. It is defined as the absence of both an S wave and a J wave. Dr. Smith studied this in 2016 and showed that TQRSD in V2 or V3 is highly specific for LAD occlusion. Here we have TQRSD in both leads in a patient with compatible symptoms. Even though the EKG looks a bit unusual, there is no question that the patient should undergo emergent coronary angiography, which is what happened in this case.
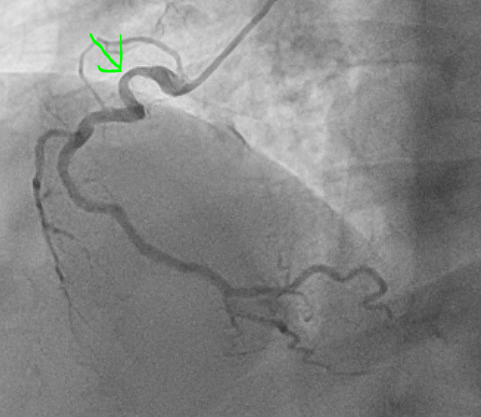
Thirty four minutes after EKG 1, the following angiogram was performed:
The angiogram was interpreted as showing a 60% stenosis in the mid LAD, 60% ostial LCx, and 50% proximal RCA. This is shown below:
LAD lesion circled in red, ramus course indicated with blue, LCx indicated in black. This is a right anterior oblique cranial view, which is best for visualizing the proximal to mid LAD.
There is no evidence of active occlusion on the angiogram. Repeat EKG following the cath is shown below. He was chest pain free at the time of the recording.
EKG 2
The TQRSD is resolved in V3 (note the reconstituted S wave) and much less pronounced in V2. The Queen of Hearts sees no OMI with high confidence. Echocardiogram performed after cath showed hyperdynamic ejection fraction with normal wall motion. Repeat cTnI obtained 3 and 6 hours after the first were 0.019 ng/mL and 0.026 ng/mL, respectively. It more than doubled, but remained within the reference range.
What does all of this mean? False positive?
This case demonstrates a few important points.
First, it is always worth remembering that coronary thrombi are dynamic. They are not set in stone. The human body has endogenous thrombolysis mechanisms. Before modern PCI, acute coronary syndrome was treated using thrombolytic drugs, which are recombinant versions of human proteins. Patients frequently spontaneously recanalize (and spontaneously reocclude). The EKG tells you what is happening in the coronary arteries right now, but that may change by the time angiography is performed, even just thirty four minutes later.
Second, there is a spectrum of causes of myocardial infarction in patients with non-obstructive coronary arteries (termed MINOCA). MINOCA is defined as myocardial infarction (detection of a rise and/or fall of cTn values with at least 1 value above the 99th percentile reference limit together with signs or symptoms of ischemia) and no angiographic stenosis >50%.
- The cTnI did not rise above the reference limit, which is required to diagnose myocardial infarction. However, the cTnI did more than double from 0.011 ng/mL to 0.026 ng/mL. changes in contemporary troponin beneath the 99% reference value may, or may not, reflect real myocardial injury. Such changes are particularly uncertain with the old "contemporary, 4th generation" troponin which does not measure delta nearly as precisely as hs troponin.
- The strict definition of MINOCA requires no stenosis > 50%, and the angiographers interpreted it as 60%. However, the difference between 50% and 60% is highly subjective to the point of being almost irrelevant, and the underlying principal applies.
- It is more of an observation than a diagnosis, per se. It requires further testing and evaluation to determine the cause and therefore treatment.
- It is not rare. A recent systematic review found that 6% of cases of acute MI are cases of MINOCA.
- The commonest causes of MINOCA include: atherosclerotic causes such as plaque rupture or erosion with spontaneous thrombolysis, and non-atherosclerotic causes such as coronary vasospasm (sometimes called variant angina or Prinzmetal's angina), coronary embolism or thrombosis, possibly microvascular dysfunction.
- Two thirds of MINOCA cases are due to atherosclerotic causes
The image on the left shows the LAD before intervention, and the red circled portion on the right indicates the stented region. It does not look that different because the lumen was not severely obstructed before.
- As always, remember that TQRSD in V2-V3 must be considered diagnostic of acute LAD occlusion.
- Occlusions can be very brief, and the finding on angiography of no occlusion does not rule out a very recent occlusion with spontaneous recanalization.
- Intravascular imaging is often necessary when conventional angiography does not provide a clear answer. Dr. Gregg Stone recently announced the results of the latest as-yet-unpublished meta analysis showing an all cause mortality benefit with the use of intravascular imaging (OCT and IVUS).
===================================
MY Comment, by KEN GRAUER, MD (12/19/2023):
- As per Dr. Frick — today's case does not technically "qualify" as MINOCA ( = MI with Non-Obstructive Coronary Arteries). That said, for practical purposes — I find it hard to think of today's case as anything but MINOCA! That's because this patient has clinical, ECG and Troponin evidence that an acute event did in fact occur (ie, a more than doubling of Troponin in the context of a changing pattern of CP that correlates temporally with "dynamic" ECG changes — therefore strongly suggesting transient acute occlusion regardless of cath findings in today's case).
- NOTE: For more on MINOCA — Please see my ADDENDUM below.
- Today's case is unique with regard to this invaluable ECG sign — in that it provides us with the opportunity to observe serial change in the relative amount of T-QRS-D (highlighted within the RED and BLUE rectangles shown in leads V2 and V3 of Figure-1).
- What ELSE changes between the time that these 2 tracings were recorded?
- Given the historical notes I include for ECG #1 and ECG #2 at the top of each of these tracings — What should the unmistakeable clinical conclusion be about what happened in today's case?
-USE.png) |
| Figure-1: Comparison of the initial ECG — with the repeat ECG done just after cardiac catheterization. What are the serial ECG changes that occur between the time that these 2 tracings were recorded? |
- Clinically — The patient's severe CP has completely resolved by the time ECG #2 is recorded!
- Upright T waves in high-lateral leads I and aVL are smaller and less "bulky" in the repeat ECG.
- Each of the inferior leads also show improvement (ie, a much smaller T wave in lead II — less T wave inversion in lead III — and less T wave inversion with resolution of the down-up biphasic T wave that was previously seen in lead aVF).
- Marked decrease in T wave amplitude in all 6 of the chest leads (especially in leads V1,V2,V3).
- CONCLUSION: Given resolution of severe CP in association with the above marked improvement of ST-T wave abnormalities in virtually all leads during the less than 1 hour between the recording of ECGs #1 and #2 — this strongly suggests reopening of a "culprit" vessel (whichever vessel this was) — regardless of the fact that "no obstructive lesion was seen" at the time the cardiac catheterization happened to be done.
- The more than doubling of Troponin (even though "normal limits" for Troponin were not surpassed) — is consistent with a brief OMI, with only a short period of total occlusion.
- It is ever-so-EASY to overlook serial ST-T wave changes if you simply review each tracing separately. Putting both tracings side-by-side (as I do in Figure-1) — tremendously facilitates and expedites recognition of "real" differences in ST-T wave morphology — which given the associated resolution of CP in this case, proves that the cause of MINOCA in today's case was indeed, a transient acute OMI.
- Had additional ECGs been done (both before and after cath) — I'm sure more developed reperfusion ST-T wave changes would have been seen.
- By way of review — I've excerpted the following material regarding T-QRS-D and MINOCA.
- As per Dr. Frick — an all-too-common misconception is that the absence of obstructive coronary disease on cardiac catheterization rules out acute coronary occlusion as the cause of the patient's acute event. This is not the case.
- To paraphrase Dr. Smith's comments in the May 19, 2020 post: — Non-obstructive coronary disease does not necessarily imply no plaque rupture with thrombus. This is because non-obstructive plaques can fissure, thrombose, totally (or near totally) occlude, have autolysis (spontaneous lysis of thrombus with reperfusion) — yet have less than 50% obstruction at angiography. These plaques will often not be recognized as "culprits", because no fissuring or ulceration is seen. As a result (and as emphasized by Dr. Frick in today's case!) — determination of plaque disruption in such patients can only be diagnosed by use of intracoronary imaging — with either higher-resolution OCT (Optical Coherence Tomography) or IVUS (IntraVascular UltraSound).
- Bottom Line: Despite lack of obstructive coronary disease on cardiac catheterization — the most common cause of MINOCA is probably still an acute OMI that spontaneously reperfused (and was no longer evident by the time cath was performed).
- The 3 most common causes of ACS (Acute Coronary Syndrome) without evidence of obstructive coronary disease on cath are: i) Myocarditis (up to 1/3 of these patients); ii) Takotsubo cardiomyopathy; and, iii) MINOCA.
- There is a trend toward these patients being younger — with a greater relative percentage of women — and fewer traditional cardiac risk factors.
- Longterm prognosis of patients with MINOCA clearly depends on the underlying etiology — but it's important to appreciate that this entity is not benign (with similar mortality as for patients with obstructive coronary disease following their infarction).
- Cardiac MRI — provides an answer to the etiology of patients with MINOCA in more than 2/3 of cases.
- Cardiac MRI successfully identifies ~80% of patients with acute myocarditis by picking up evidence of inflammation — with the distinct advantage of being noninvasive compared to endomyocardial biopsy.
- Use of LGE (Late Gadolinium Enhancement) — is routinely recommended with cardiac MRI to increase diagnostic yield, as a means to identify fibrosis and other abnormalities in cardiac tissues.
- Cardiac MRI (especially with the addition of LGE) provides insight to longterm prognosis of patients with MINOCA.
-USE.png) |
| Figure-2: Classification of Underlying Diagnoses in Patients with MINOCA (Adapted from Table-1 in Sykes et al: Interventional Cardiology Review: 16:e10, 2021). NOTE: As per Sykes et al — The entities listed under "Other Etiology" may be diagnosed following further investigation and should be considered separately (because they are typically associated with myocardial injury but not considered an MI by the 4th universal definition of MI). This is an important indication for cardiac MRI in patients suspected of MINOCA. |
- T-QRS-D — is defined as the absence of both a J-wave and an S-wave in either lead V2 or lead V3. Although simple to define — this finding may be subtle! I fully acknowledge that it has taken me a while to become comfortable and confident in its recognition.
- NOTE: In the "right" clinical setting — Drs. Smith and Meyers have on occasion accepted T-QRS-D as a suggestive finding of acute OMI if present in lead V4. Validation of this ECG marker is less secure in leads other than V2,V3,V4.
- TOP in Figure-3 — Despite marked ST elevation in this lead V3 — this is not T-QRS-D, because there is well-defined J-point notching (BLUE arrow). This patient had a repolarization variant as the reason for ST elevation.
- BOTTOM in Figure-3 — This is T-QRS-D, because in this V3 lead there is no J-point notching — and, there is no S wave (RED arrow showing that the last QRS deflection never descends below the baseline).
 |
- Isn’t it tempting to say there is T-QRS-D in the initial ECG that was done in the ED ( = ECG #1 in Figure-4)? After all, there is no S wave in lead V3 ...
 |
Figure-4: I've labeled the first 2 ECGs shown in the November 14, 2019 post (See text). |
- The reason the ST-T wave appearance in lead V3 of ECG #1 does not qualify as T-QRS-D — is that despite lack of an S wave in this lead, there is J-point notching (or at least J-point slurring) that is characteristic of repolarization variants.
- Other ECG features in ECG #1 in favor of a repolarization variant instead of acute OMI include: i) A relatively short QTc interval and tall R waves in the mid-chest leads; ii) Lack of reciprocal ST depression; iii) A similar “look” to the peaked T waves that we see in at least 9 of the 12 leads in ECG #1 (compared to a more localized ST-T wave picture that is typical with acute infarction); and, iv) J-point notching or slurring that is typical for repolarization variants in no less than 7 of the 12 leads in ECG #1 (BLUE arrows in Figure-4).
- Therefore — more information was needed to attain greater certainty (ie, stat Echo looking for wall motion abnormality; additional ECGs on this patient; serial troponins).
- In this case — finding a prior ECG on this patient from a year earlier was revealing (ECG #2 in Figure-4). Neither the lack of S wave in lead V3, nor J-point notching or slurring were new findings (RED arrows in ECG #2). This confirmed the impression that the ST-T wave appearance in ECG #1 reflected a longterm repolarization variant in this patient.
- P.S.: Given that there is a very small-but-present percentage of lethal cardiac arrhythmic events in otherwise healthy young adut individuals who have repolarization variants — I no longer use the term, "BER" (Benign Early Repolarization) when I see this type of ECG finding (Zakka & Refaat-ACC, 2016). Instead, I simply call it "repolarization variant" — because this ECG finding is usually, but not 100% "benign". IF a patient with the ECG findings in ECG #1 presented with new, cardiac-sounding syncope — full evauation would be in order.








No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.