A 70-something female with no previous cardiac history presented with acute chest pain.
She awoke from sleep last night around 4:45 AM (3 hours prior to arrival) with pain that originated in her mid back. She stated the pain was achy/crampy.
Over the course of the next hour, this pain turned into a pressure in her chest. She said this was midsternal and felt like a tightness. This originally radiated into her left arm. Over some time and the pain moved into her other arm as well as her jaw. She also had some shortness of breath.
She was brought in by ambulance and received aspirin and nitroglycerin en route. She stated that this did provide minimal relief.
She continues to have pressure that radiates to her right arm and the left side of her neck. She has no previous cardiac history of which she is aware
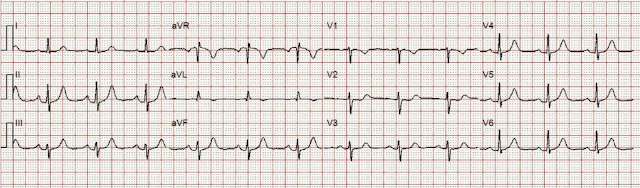
911 was called and here is her Prehospital ECG:
What do you think?
There are inferior and lateral T-waves that could be hyperacute, and I only know they are for certain because of the ST depression in V2: along with these T-waves, this STD is diagnostic of posterior OMI.
The Queen of Hearts says so too:
The Queen of Hearts PM Cardio App is now available in the European Union (CE approved) the App Store and on Google Play.
For Americans, you need to wait for the FDA. But in the meantime:
Case continued
She arrived in the ED and here is the first ED ECG. I don't know if her pain was getting better or not.
The downsloping ST depression is very suspicious for OMI, but not diagnostic. In comparison with the first ECG, I would guess that the artery is reperfusing.ED at 3.5 hours
The T-waves have become smaller, but there is persistent STD in V2. This is OMI until proven otherwise.ED at 3.75 hours
ED at 5.5 hours
The patient went for an angiogram at about 7 hours after arrival.
Angiogram
No obstructive epicardial coronary artery disease
Cannot exclude non-ACS causes of troponin elevation including coronary vasospasm, stress cardiomyopathy, microvascular disease, etc.
Echo
The estimated left ventricular ejection fraction is 56 %.
New Wall motion abnormalities:
Regional wall motion abnormality-anterolateral.
Regional wall motion abnormality-inferolateral (distal).
Post cath ECG:
No further troponins were measured, but it looks like there is recurrent OMI
Next day: A CT Coronary Angiogram was done (CTCA)
CARDIAC MORPHOLOGY AND FUNCTION:
1. Left ventricular end-diastolic volume: 99 ml.
2. Left ventricular systolic volume: 49 ml.
3. Stroke-volume:50 ml.
4. Left ventricular ejection fraction:50 %.
5. Wall motion, wall thickening: No wall motion abnormality detected.
Normal wall thickness. Region of hypoperfusion involving the anterior
lateral left ventricular wall extending into the papillary muscles, best
evidenced on the delayed series.
CORONARY ARTERIES:
Exam was not directly tailored for coronary artery evaluation, noting
recent diagnostic coronary angiogram. The LAD, circumflex, and RCA appear
patent. The distal LAD tapers relatively abruptly, but no focal vessel cut
off is identified.
IMPRESSION:
1. Regional hypoperfusion involving a portion of the anterolateral left
ventricular wall and the papillary muscles.
2. No wall motion abnormalities detected. Ejection fraction calculated at 50%.
3. Detailed coronary artery evaluation not performed.
Exam was interpreted and discussed with Dr. Simegn of cardiology, who reviewed the cardiac portion.
This suggests further severe ischemia. It would appear as if troponins would have climbed much higher. And yet the arteries remain open.
Is this small vessel obstruction? Downstream vasospasm?
Unable to get MRI due to claustrophobia
Cardiology note:
Overall picture is consistent with diagnosis of MINOCA and therefore will treat with DAPT x1 month along with other guideline directed medical therapy as below
NSTEMI -- MINOCA (myocardial infarction with nonobstructive coronary arteries)
- TTE performed this morning, anterolateral wall motion abnormality
- Isosorbide dinitrate 20 mg tid - may discontinue give evidence for MINOCA
- Metoprolol
- Atorvastatin 20 mg daily
- Clopidogrel 75 mg daily
- Start ASA 81 mg daily
- Goal K ~4.5, Mg ~2.5
Acute thrombosis at the site of non-obstructive eccentric plaque thrombosis — Many atherosclerotic plaques expand outward rather than encroaching on the arterial lumen. These ”positively-remodelled” plaques are often lipid rich and have a thin fibrous cap; they are vulnerable to rupture into the lumen [1,9,10]. Transient and partial thrombosis at the site of a non-obstructive plaque with subsequent spontaneous fibrinolysis and distal embolization may be one of the mechanisms responsible for the occurrence of MINOCA. Similarly, coronary erosion with loss of surface endothelium, possibly due to hyaluronan and neutrophil accumulation, can also cause MINOCA [1,11]. (See "Mechanisms of acute coronary syndromes related to atherosclerosis".)
The reason for these cases to be labeled as MINOCA is that angiography is of limited utility for the purpose of elucidating plaque-related thrombosis as a cause of thrombosis due to its low resolution as well as the fact that it does not interrogate the lumen of the vessel. Thus, intracoronary imaging modalities are crucial in this setting. Plaque rupture or erosion has been diagnosed by intravascular ultrasound in about 40 percent of women with MINOCA [12]. Optical coherence tomography, due to its high resolution, may provide additional information [10,13].
As MINOCA is associated with a risk of recurrent cardiovascular events over time, comparable with that of patients with acute coronary syndromes (ACS) and obstructive atherosclerosis [5,14,15], these patients require dual antiplatelet treatment for 12 months and statins. In particular, long-term lipid-lowering therapy with statins after MI is associated with a significant increase of the fibrous-cap thickness, paralleling the reduction of the lipid content of the plaque [16]. (See "Prevention of cardiovascular disease events in those with established disease (secondary prevention) or at very high risk".)
From Gue at al.
STEMI MINOCA versus NSTEMI MINOCA
STEMI occurs in the presence of transmural ischaemia due to transient or persistent complete occlusion of the infarct-related coronary artery. In patients presenting with non-ST-segment elevation MI (NSTEMI), the infarct is subendocardial. This pathophysiological difference also seems to be present within the MINOCA cohort. Registry data indicate that 6–11% of patients with acute MI have nonobstructive coronary arteries. Within the literature, MINOCA tends to present more commonly as NSTEMI than STEMI: the incidence of MINOCA reported in patients presenting with NSTEMI is about 8–10% and in STEMI cohorts it is 2.8–4.4%. This has resulted in an under-representation of STEMI MINOCA patients in the literature. Most studies examine undifferentiated ACS cohorts, with only a handful providing separate data. These studies indicate that the 1-year mortality of MINOCA presenting as STEMI is 4.5%, in contrast to the mortality of unselected MINOCA ACS patients who have a mortality of 4.7%. The underlying aetiology of MINOCA is similar among those presenting with STEMI and in all-comer MINOCA patients with ACS, with non-coronary aetiology responsible for presentation in 60–70% of individuals with STEMI and in 76% of unselected ACS patients.
References:
1. Lindahl B, Baron T, Erlinge D, et al. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation [Internet] 2017;135(16):1481–9. Available from: http://dx.doi.org/10.1161/CIRCULATIONAHA.116.026336 https://www.ahajournals.org/doi/epdf/10.1161/CIRCULATIONAHA.116.026336
2. Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): The Past, Present, and Future Management [Internet]. Circulation. 2017;135(16):1490–3. Available from: http://dx.doi.org/10.1161/CIRCULATIONAHA.117.027666 https://www.ahajournals.org/doi/epdf/10.1161/CIRCULATIONAHA.117.027666
3. Gue YX, Kanji R, Gati S, Gorog DA. MI with Non-obstructive Coronary Artery Presenting with STEMI: A Review of Incidence, Aetiology, Assessment and Treatment. Eur Cardiol [Internet] 2020;15:e20. Available from: http://dx.doi.org/10.15420/ecr.2019.13
4. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med [Internet] 2013;368(21):2004–13. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23697515
Read more about MINOCA at this post:
Hyperacute T-waves -- missed. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA) may be due to transient thrombotic Occlusion MI.
MY Comment, by KEN GRAUER, MD (11/16/2023):
I suspect that the entity known as MINOCA (MI with Non-Obstructive Coronary Arteries) — is not fully appreciated by many clinicians.
- I was surprised to learn that an estimated 5-15% of patients (depending on the studied population) who are diagnosed with either STEMI or NSTEMI — turn out to have MINOCA, in which cardiac catheterization fails to reveal any infarct-related artery (ie, no "culprit" vessel with at least 50% stenosis) — and no clear systemic etiology is found to explain the patient's presentation to the hospital (Tamis-Holland et al: AHA Scientific Statement on MINOCA — Circulation 139:e891-e908, 2019 — Broncano et al — RadioGraphics 41:8-31, 2021 — and — Sykes et al — Interventional Card Review 16:e10, 2021).
- Echo — showed regional wall motion abnormalities in the anterolateral and inferolateral regions.
- CTCA — showed a region of hypoperfusion involving the anterior lateral LV wall, extending into the papillary muscles (best evidenced on the delayed series).
- Unfortunately — Cardiac MRI could not be done due to claustrophobia.
- Given this patient's presentation for new CP — I immediately knew on seeing her initial ECG, that prompt cardiac cath was indicated. For clarity in Figure-1 — I've reproduced that initial tracing.
- That said — I thought there were some unusual features in ECG #1 that are worthy of mention.
-USE.png) |
| Figure-1: The initial ECG in today's case. |
- Q waves — A tiny (probably not significant) q wave is seen in lead aVL.
- R wave progression — is normal (with transition, where the R wave becomes taller than the S wave is deep — occurring normally, here between leads V3-to-V4).
- Hyperacute T waves are seen in 6 leads! Specifically — T waves are peaked and disproportionately tall in leads II,III,aVF — and, in leads V4,V5,V6. ST segments are straightened to varying degree in these 6 leads — which makes for an "eye-catching" abnormal angulation at the juncture between ST segments and the beginning of the T wave in these 6 leads.
- There is subtle ST segment flattening in lead I.
- The tiny amplitude QRS in lead aVL manifests a small, narrow q wave — a hint of ST segment coving without elevation — and shallow T wave inversion that presumably is abnormal given the predominantly upright QRS.
- The T wave in lead V1 may normally be inverted — but usually not with this amount of ST segment coving, nor with this depth of T wave inversion. This is subtle and nonspecific — but probably not "normal".
- There is 1 mm of ST segment elevation in lead aVR — which in the context of ST segment flattening in most other leads, suggests that there may be a component of subendocardial ischemia from underlying coronary disease.
- The above said — the most striking abnormalities in these remaining 6 leads are the ST-T waves in leads V2,V3 that suggest posterior OMI has recently occurred. Note especially the straight (shelf-life) ST segment flattening (albeit with at most, no more than a tiny amount of ST depression) — in both of these leads that typically manifest at least a slight amount of gentle upsloping ST elevation as a normal finding. This is followed by terminal T wave positivity, with T waves that appear slightly "hypervoluminous" in leads V2,V3 given tiny size of the R waves in these leads.
- In a patient with new CP — there is no doubt that the ST-T waves in 6 leads (II,III,aVF; V4,V5,V6) have to be interpreted as "hyperacute" — which of itself is indication for prompt cath.
- In a patient with new CP — the above described ST-T wave appearance in leads V2,V3 has to be interpreted as suggestive of posterior OMI until proven otherwise. That said — these ST-T wave changes in leads V2,V3 looked "less acute" than did the T waves in the inferior and lateral chest leads.
- Given the patient's older age + the combination of diffuse ST segment flattening + ST elevation in lead aVR — I wondered about a possible "background" of multi-vessel coronary disease.
- Bottom Line: I didn't think this overall ECG picture produced any unifying diagnosis. That said — I knew prompt cath was indicated. In the "retrospectoscope" — this lack of a unifying ECG picture may reflect a more diffuse underlying process at the etiology for this patient's MINOCA.
- An all-too-common misconception is that the absence of obstructive coronary disease on cardiac catheterization rules out acute coronary occlusion as the cause of the patient's acute event. This is not the case.
- To paraphrase Dr. Smith's comments in the May 19, 2020 post: — Non-obstructive coronary disease does not necessarily imply no plaque rupture with thrombus. This is because non-obstructive plaques can fissure, thrombose, totally (or near totally) occlude, have autolysis (spontaneous lysis of thrombus with reperfusion) — yet have less than 50% obstruction at angiography. These plaques will often not be recognized as "culprits", because no fissuring or ulcertaion is seen. As a result — determination of plaque disruption in such patients can only be diagnosed by use of intracoronary imaging — with either higher-resolution OCT (Optical Coherence Tomography) or IVUS (IntraVascular UltraSound).
- Bottom Line: Despite lack of obstructive coronary disease on cardiac catheterization — the most common cause of MINOCA is probably still an acute OMI that spontaneously reperfused, and was no longer evident by the time cath was performed.
- The 3 most common causes of ACS (Acute Coronary Syndrome) without coronary disease are: i) Myocarditis (up to 1/3 of these patients); ii) Takotsubo cardiomyopathy; and, iii) MINOCA.
- There is a trend toward these patients being younger — with a greater relative percentage of women — and fewer traditional cardiac risk factors.
- Longterm prognosis of patients with MINOCA clearly depends on the underlying etiology — but it's important to appreciate that this entity is not benign, with similar mortality as for patients with obstructive coronary disease following their infarction.
- Cardiac MRI — provides an answer to the etiology of patients with MINOCA in more than 2/3 of cases.
- Cardiac MRI successfully identifies ~80% of patients with acute myocarditis by picking up evidence of inflammation — with the distinct advantage of being noninvasive compared to endomyocardial biopsy.
- Use of LGE (Late Gadolinium Enhancement) — is routinely recommended with cardiac MRI to increase diagnostic yield, as a means to identify fibrosis and other abnormalities in cardiac tissues.
- Cardiac MRI (especially with the addition of LGE) provides insight to longterm prognosis of patients with MINOCA.
-USE.png) |
| Figure-2: Classification of Underlying Diagnoses in Patients with MINOCA (Adapted from Table-1 in Sykes et al: Interventional Cardiology Review: 16:e10, 2021). NOTE: As per Sykes et al — The entities listed under "Other Etiology" may be diagnosed following further investigation and should be considered separately (because they are typically associated with myocardial injury but not considered an MI by the 4th universal definition of MI). This is an important indication for cardiac MRI in patients suspected of MINOCA. |








No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.