Written by Jesse McLaren, with comments from Smith and Grauer
A 60 year old presented with three weeks of intermittent non-exertional chest pain without associated symptoms. ECG was labeled ‘normal’ by the computer (confirmed by the overreading cardiologist) and the high-sensitivity Troponin I was normal at a value of 11 ng/L (Abbott Alinity assay, where normal is <26 in males, <16 in females; this assay is nearly identical to the Abbott Architect high sensitivity assay). So the patient was low risk according to HEART and EDACS scores. Should this patient be discharged home? How about a stress test?
(Marqette 12SL algorithm)
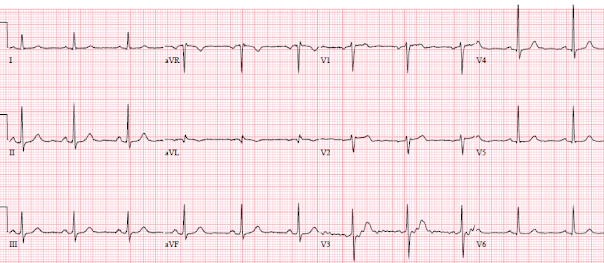
There’s sinus bradycardia with normal conduction, normal axis, normal R wave progression, and normal voltages. There are biphasic T waves in III/aVF with reciprocal down/up T wave in aVL, and a disproportionately tall T wave in V2. This is diagnostic of inferoposterior reperfusion.
Comparison to the old ECG below (from 4 years prior) confirms that all these changes are new (and can’t be explained by the slight shift in axis in the frontal plane, or lead placement of V2):
If these new primary biphasic T waves were in the anterior leads, in a patient with resolved chest pain, this might have been recognized as “Wellens syndrome”, associated with critical LAD stenosis. This would have led to an admission for angiogram regardless of the atypical pain or negative troponin—or could even have led to cath lab activation, as “Wellens” is often called a “STEMI-equivalent” even though “STEMI” implies occlusion and “Wellens” implies reperfusion (the artery is open). But because they were in the inferoposterior leads they were missed.
Smith comment: What does Wellens' syndrome mean? As Jesse said, this is inferoposterior Wellens'. Such T-wave inversion happens identically in other myocardial territories than the anterior wall (LAD, Wellens'). It means that, when the patient had active chest pain, there was unrecorded STEMI/OMI that then spontaneously reperfused. This is a fact, although I know of no literature discussing it. As an example, see this case: First ED ECG is Wellens' (pain free). What do you think the prehospital ECG showed (with pain)?
Miss #1: using ED risk stratification tools in the presence of a diagnostic ECG
The emergency physician interpreted the ECG as “nonspecific
inferior repolarization abnormalities”. Based on this interpretation, in
combination with atypical chest pain and a negative troponin, the patient had
low HEART and EDACS scores so they were discharged on aspirin with outpatient
cardiology follow up. However, this ECG is VERY specific for Wellens' pattern ECG, and fits with Wellen's syndrome; therefore it is SPECIFIC for acute MI
The HEART
(1) and EDACS
(2) scores were designed to risk stratify undifferentiated chest pain patients
in the ED, in order to identify those at low risk for Major Adverse Cardiac
Events (MACE) who are safe for discharge. But as HEART score creator Dr. Backus
explained on MDCalc,
“a minor pitfall is that the user needs at least some experience taking a chest
pain history and reading an ECG to interpret these two elements of the score.” Both
scores are based on a limited interpretation of the ECG: the HEART score
stratifies ECGs into “normal”, “non-specific repolarization” and “significant
ST-segment deviation”, and patients are deemed low-risk if they have a total
score <4 regardless of the ECG component; the EDACS score classifies
patients at low risk if they have a score <16, a negative troponin and an
ECG with “no new ischemic changes.”
Patients with STEMI are obviously excluded, because the
patient is no longer undifferentiated and no longer needs to be risk stratified
for future risk of MACE: the ECG has already differentiated them by diagnosing acute
coronary occlusion. The same exclusion should also apply to patients with ECGs diagnostic of STEMI(-)OMI or
reperfusion, even if the ECG is labeled as “normal” (3). This is even more important for those using the HEAR score without troponin, for patients who are low risk based on the other components.(4)
Miss #2: 'normal troponin' - conflating undetectable and 99% percentile
The EDACS and original HEART scores called for a repeat troponin level,
which in this patient might have been elevated and led to hospital
admission. Many centres now use the modified HEART score that only
uses one high-sensitivity troponin level, but this must be under the level of detection, or under some validated pre-specified very low lever at least three hours after chest pain onset. But with the pressure to discharge patients early there is a shift towards using any single troponin in the 'normal range.'
In this case, the troponin level was 11 and interpreted as 'normal' because it is below the 99th percentile (<26 in males, <16 in females). But it was above the level of detection of this assay (<2). In a prospective cohort study of 6304 patients of whom 782 had type 1 MI, a troponin level of 11 was only 93% sensitive at excluding MI.(5)
Smith comment: A single troponin value may ONLY be used if below some extremely low value that is far below the 99th percentile URL. At our institution, we have the Abbott Architect hs cTnI (which is similar to Abbott Alinity). For this assay, the 99th percentile URL is 16 ng/L for women and 34 for men; the Limit of Detection is 1.9 ng/L in Europe and 3.9 ng/L by US FDA. The literature supports rule out with a single value below 5 ng/L if drawn at least 3 hours after the onset of pain (5). The studies which support using the HEART and EDACS and a single troponin also require that it be below some very low value far below the 99th percentile, but that value must be ascertained by studies of the specific assay.
So it is a major error to discharge this patient based on 1 troponin below the 99th percentile. Other studies show that, regardless of time of blood draw, if the value is below the Limit of Detection (LoD) of the Abbott Architect (outside the US, 1.9 ng/L), there is a greater than 99% NPV (9, 10)
Miss #3: using stress testing for a critical stenosis predicted on ECG
A few days after the discharge the patient saw cardiology, who interpreted the ECG as “sinus rhythm with nonspecific ST changes inferiorly.” So they scheduled a stress test for a week later. This is a very risky move as stressing a heart with a critical and dynamic stenosis can induce myocardial infarction(6). Interestingly, while the guidelines list "high-risk unstable angina" as a clinical contraindication for stress testing, they don't mention Wellens' or any ECG contraindications to stress testing(7), though some stress tests have been avoided by identifying this pattern(8).
The patient's stress echo showed inducible ischemia, with basal inferior wall hypokinesis (corresponding to the ECG changes). Luckily they had no complication during the stress test, or during the next two weeks awaiting for the elective angiogram.
A month after their initial ED visit, with increasing episodes of chest pain, the patient had their angiogram which found an 80% circumflex lesion (corresponding to the ECG changes), which was stented. Below is the discharge ECG, showing evolving reperfusion, with deeper T waves inferiorly and taller T wave in V2
This patient is incredibly lucky that they did not re-occlude for any length of time after this first ECG between the first ECG and the angiogram a month later.
Here’s another lucky patient, with a similar story and ECG: 60 year old with three weeks of exertional chest pain, who was pain free on arrival in the ED. Below is their old ECG (from two months prior) and triage ECG when pain free, both labeled “normal” by the computer and the over-reading cardiologist:
(Marqette 12SL algorithm)
Again there is new primary T wave inversion in the inferior leads, and a taller T wave in aVL. There is also new early R wave progression with R>S in V2.
These were recognized by the physician reading triage ECGs before seeing the patient: here's a photocopy of the original triage ECG above to show that the physician identified inferoposterior reperfusion in real time, and that these signs can be learned
The patient was referred to cardiology but admitted as "NSTEMI" and had an angiogram 3 days later which found a 95% RCA occlusion. Fortunately it hadn't re-occluded for any length of time between admission and angiogram, with a peak Trop I of 1000 ng/L (normal <26 in males, <16 in females).
But these cases show the potential dangers of delayed recognition and treatment of inferior reperfusion
Take away
1. The HEART and EDACS scores are helpful to risk stratify
patients with chest pain, but they hinge on accurate ECG interpretation: a low score doesn’t apply if the ECG shows STEMI(+)OMI, and shouldn’t be used for
STEMI(-)OMI or OMI reperfusion either
2. The pressure to discharge patients early can lead to relying on single troponin levels below the 99th percentile, and using terms like 'normal troponin' or 'negative troponin' conflates this with below the level of detection. Specifying the level is more accurate, evidence-based and safe
3. Rather than using terms like “STEMI” and “Wellens”, it’s more helpful to describe the underlying pathology and ECG pattern pattern: Occlusion MI, and reperfusion T wave inversion
4. Learn the signs of reperfusion: as Dr. Smith says, it’s not the ECG that is nonspecific; it is the interpreter
5. Spontaneous reperfusion is at risk for spontaneous re-occlusion: these patients need aggressive management and urgent angiography, not stress testing
6. ECG’s can be labeled as ‘normal’ by the computer (and confirmed by cardiology) even with diagnostic signs of occlusion or reperfusion
References
1. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol 2013
2. Shin YS, Ahn S, Kim YJ. External validation of the emergency department assessment of chest pain score accelerated diagnostic pathway (EDACS-ADP). Am J Emerg Med 2020
3. Bracey A, Meyers HP, Smith SW. Emergency physicians should interpret every triage ECG, including those with a computer interpretation of “normal.” American Journal of Emergency Medicine 2022
4. Moumneh T, Sun BC, Baecker A, et al. Identifying patients with low risk for acute coronary syndrome without troponin testing: validation of the HEAR score. Am J Med 2021
5. Shah ASV, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015
6. Patel J, Alattar F, Koneru J, et al. ST-elevation myocardial infarction after pharmacologic persantine stress test in a patient with Wellens’ syndrome. Case Rep Emerg Med 2014
7. Gulati M, Levy P, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines JACC 2021
8.
Yazdi D, Sharim J. A nearly stressful
situation—a case of Wellens syndrome. JAMA Intern Med 2019
9. Sandoval Y, Smith SW, Love SA, Sexter A, Schulz K, Apple FS. Single High-Sensitivity Cardiac Troponin I to Rule Out Acute Myocardial Infarction. Am J Med [Internet] 2017;130(9):1076–83.e1. Available from: http://dx.doi.org/10.1016/j.amjmed.2017.02.032
10. Greenslade J, Cho E, Van Hise C, et al. Evaluating Rapid Rule-out of Acute Myocardial Infarction Using a High-Sensitivity Cardiac Troponin I Assay at Presentation. Clin Chem [Internet] 2018;64(5):820–9. Available from: http://dx.doi.org/10.1373/clinchem.2017.283887
===================================
- The reason it's important to go through this soul-searching process — is so that we can learn from mistakes made, in order to optimize care in the future.
- Great point by Drs. Smith and McLaren to emphasize that “Wellens’ Syndrome” does not only occur in the anterior leads. Although the distribution of the LAD is the most common place to see the reperfusion ST-T wave changes of Wellens’ Syndrome — this 1st Case illustrates what an Infero-Postero Lead Wellens’ Syndrome looks like.
- KEY Point: The history we were told in Case #1 was “3 weeks of intermittent non-exertional chest pain without associated symptoms”. But not only was the initial ECG misinterpreted — the fact that the history was summarized as “3 weeks of intermittent chest pain” indicates that the treating clinicians failed to understand both the expected ECG findings, as well as the required time course for symptoms when there is a process such as occurs with true "Wellens' Syndrome".
- This is relevant — because it invalidates the “History” component of the HEART and EDACS scores that were said to be "low risk". As per Drs. McLaren and Smith — the initial ECG in Case #1 shows that an event did occur. The question is WHEN? Although diagnostic — ECG changes in the initial tracing in Case #1 (as well as the History) suggest days, or even up to 2-3 weeks earlier might be the timing of the event — in which case Troponin should not necessarily be expected to be elevated.
- WHAT brought this 1st patient to the ED on the day he presented — which was no less than 3 weeks after the onset of his symptoms? While fully realizing that we can't write a "book-long history" on all patients — striking for its absence is any mention in the History regarding symptoms on the day he presented (and at the time the initial ECG was obtained). Optimal clinical ECG interpretation depends on correlation with the timing, duration and severity of symptoms.
- I always believed not to do a low-level ETT after a completed (stable) MI under observation (ie, in the hospital) before the patient goes home — is to allow the patient to do their own "unmonitored Stress Test” once they get home.
- That said — my experience having taught ambulatory Stress Testing for 3 decades, has always been that the most difficult part of correctly performing an ETT — is knowing the extent to “push” (encourage) the patient — and — learning WHEN to stop the test. I’ve always been impressed at how quickly a patient can go from easily tolerating activity on the treadmill — to virtual collapse no more than moments later.
- So — performing an ETT on this patient in Case #1 would not necessarily be contraindicated IF the test was performed with the proper caution (which in Case #1 means fully appreciating that this patient has had a recent event!).
- Concerning for its absence in the History we were given is any mention about WHEN during the Stress Echo (ie, at what time and at what activity level during the test) was “inducible ischemia” seen? Was this objective evidence of inducible ischemia accompanied by chest pain?
- If objective ECG and Echo evidence of induced ischemia occurred within the first few minutes of low-level exercise — then even IF the clinicians failed to appreciate that this patient’s initial ECG was diagnostic for a recent event — there is NO WAY this patient should have had to wait 2 weeks for an “elective angiogram”. The cardiologists I used to work with would have admitted that patient for cath the next day.
- And IF it turned out that despite inducible ischemia (on Echo and ECG) at low-level exercise, that this patient did not have any chest pain — then this is the definition of “Silent" Ischemia. It might explain why this patient took 3 weeks to show up in the ED in the first place (because at least some — or perhaps most of the time, the patient may have been unaware of symptoms during activities of daily living). In that case — what might happen with all of the inducible “silent” ischemia episodes (ie, without symptoms) that would be bound to occur over the 2 weeks until the “elective” cath was finally done?
To Be CLEAR: I am not saying that a Stress Test should have been done on this patient in Case #1. There should have been better appreciation of the History to be looked for — and ECG reperfusion changes from recent infarction should have been recognized — such that optimal care would have entailed prompt cath with a preventive intervention. I am simply saying that when done properly — Stress Testing can be safe done 10 days after a stable MI.
=====================
- Details of the History are again lacking — because an ECG had been obtained 2 months prior, yet we do not know the reason WHY this prior ECG was done.
- KEY Point: Without knowing anything about this 60-year old patient's prior medical history — the story we are given (ie, 3 weeks of exertional chest pain — but painfree on presenting to the ED) — provides the definition of angina pectoris. And IF this is the 1st time that this 60-year old is having exertional chest pain — then this fits the definition of "unstable" angina.
- But I wanted to comment on this 2nd patient's initial ECG — which apparently was read as "normal" (Figure-1).
-USE%20copy.png) |
| Figure-1: I have reproduced and labeled the 4th ECG shown in Today's Blog Post. This ECG is from Case #2. It was obtained ~2 months prior to this patient's presentation in the ED. |
- The rhythm is sinus at ~65/minute. Intervals (PR, QRS, QTc) and the axis is normal. There is no chamber enlargement.
- Regarding Q-R-S-T Changes — There is a tiny q wave in lead aVL. R wave progression is normal. It is the ST-T wave changes that are of concern.
- The normal ST segment manifests a gentle upsloping until it blends almost imperceptibly into the T wave. Instead — there is subtle-but-real ST segment straightening in multiple leads in Figure-1 (schematically shown by the parallel RED lines).
- If anything — there is usually slight (normal) ST elevation in leads V2 and V3. Not only is this normal slight amount of ST elevation missing in these 2 leads — but J-point ST depression is present beginning in lead V3 — and persisting to lead V6 (BLUE arrows).
- We were not told whether this patient was having symptoms at the time the ECG in Figure-1 was obtained. But we know that he had typical angina 5 weeks later, which then persisted for 3 more weeks until he finally presented to the ED.
- There is an "art" to eliciting the History. There are many ways to ask the questions. IF this patient in Case #2 was in fact having typical anginal symptoms at the time the ECG in Figure-1 was obtained — this should have prompted at the very least some Stress Test (if not cardiac cath) — which if done, could possibly have prevented the large infarction he subsequently had.





No comments:
Post a Comment
DEAR READER: I have loved receiving your comments, but I am no longer able to moderate them. Since the vast majority are SPAM, I need to moderate them all. Therefore, comments will rarely be published any more. So Sorry.