This case was sent by a former HCMC resident:
A 20-something non-English speaking woman with a history of some sort of congenital heart defect collapsed at home and EMS found her with a regular wide complex tachycardia around 200 bpm. They attempted cardioversion with
adenosine, unsuccessfully (dose was uncertain, thought to be 12 mg, but this would be an unusual initial dose).
She was cyanotic and minimally responsive on arrival here, and had a healed sternotomy scar. There were no records immediately available.
Her blood pressure was normal throughout the case. O2 sats were 70s and 80s on high flow O2, but there was no evidence of pulmonary edema. They did notice "clubbing" of the fingers.
A relative was able to state in broken English "single ventricle."
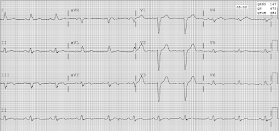
Here is the initial 12-lead ECG:
 |
There is a regular, wide complex tachycardia at a rate of 160, with no P-waves.
There is a monomorphic Right Bundle Branch block pattern with QRS duration of between 140 - 160 ms (is it difficult to ascertain the exact beginning and end of the QRS)
Is it Ventricular Tachycardia (VT) or SVT with aberrancy?
What else can be said about it?
What should be done?
Full interpretation is at the far bottom of the post |
Comment:
If the patient is unstable, just cardiovert with electricity. Her altered mental status may be due to hypoperfusion (shock), in spite of the normal blood pressure (non-invasive blood pressures are unreliable in sick patients). Thus she must be considered unstable, so use
electricity!
One might call this patient unstable because of the low saturations, but a patient with a known single ventricle is likely to have cyanotic heart disease and to have
baseline O2 saturations that are very low, even on high flow O2. This is especially true if there is no evidence of pulmonary edema on chest X-ray or bedside ultrasound. Clubbing is further supportive evidence.
Thus, it is very likely that the patient has
uncorrectable hypoxemia due to shunt physiology.
If the patient had been stable (conscious), then there would have been a few minutes to think:
First, if one can easily find old ECGs that are in sinus rhythm, then one can compare the QRS morphology at baseline with this one in tachycardia. If identical, then this is supraventricular (which includes sinus tachycardia). To diagnose sinus tachycardia,
one can use Lewis leads in order to uncover hidden P-waves. This takes about 30 seconds.
Here are the instructions for recording Lewis leads. It is done with the monitor, not the 12-lead.
Second, in most young people without structural cardiac disease, SVT is more likely. However, this patient does have structural heart disease, so
VT is not at all unlikely.
Third, assuming it is truly NOT sinus tachycardia, either adenosine at a higher dose or electrical cardioversion works well. Even if it is sinus tach, neither of these therapies is terribly dangerous, but they will not benefit the patient.
Fourth, is there any evidence from the QRS that this is SVT vs. VT? There is a "northwest" axis (between -90 and 180, with a large monophasic R-wave in aVR). This implies origination at the apex (the ventricle, where VT originates) with propagation towards the base of the heart ("base" meaning upper right). On the other hand,
the first part of the QRS (initial depolarization) is very rapid, even in that upright R-wave in aVR.
Look at lead V5: that first part is comprised by the onset of the R-wave to nadir of the S-wave, and it is less than 60 ms. Thus, SVT with aberrancy is not at all unlikely.
Thus, in a stable patient: As it is easiest, I would try a larger dose of adenosine.
Case continued:
"We intubated the patient, then consulted cardiology and decided to do an electrical cardioversion at 200J biphasic. This was successful."
"I found records which were limited but suggested congenital heart deformity, and that her baseline O2 sats were in the 70-80% range."
Here is the post cardioversion ECG:
 |
| What does this post cardioversion ECG tell you about the initial rhythm? |
There is clearly sinus rhythm, with exactly the same QRST morphology as the first.
This proves that the original was indeed SVT with aberrancy.
Airway, Breathing, Circulation (ABC)
We are always taught to use this sequence, but we often get it confused. Sometimes the issue with the airway and breathing is really a circulation problem (shock with altered mental status). I would cardiovert first, which will usually solve the problem. Intubate if it does not. Also, when the patient is obtunded (stuporous), it is both dangerous and unnecessary to sedate before cardioverting. Our research at HCMC shows these critically ill patients do not remember such events.
A Primer on Wide Complex Tachycardia
First, use electricity on unstable patients.
What is instability? Severe shock, cardiac ischemia, or pulmonary edema.
If stable, you can take a bit of time to think.
Differential Diagnosis of Wide Complex Tachycardia
When assessing for the rhythm in wide complex regular tachycardia, these are the assessments I make, though no method is foolproof:
--Sinus with aberrancy -- Aberrancy can be due to toxins (wide complex from the many drugs which have sodium channel blocking effects and prolong the QRS) or to hyperkalemia.
--SVT with aberrancy. This can be either 1) AVNRT with abnormal conduction to the ventricles, or 2) antidromic AVRT (AV reciprocating tachycardia) with the impulse going down an accessory pathway and up through the AV node)
--Ventricular Tachycardia (VT)
Assess pretest probability:
--Majority of wide complex tachycardia is VT
--If h/o MI, cardiomyopathy, low Ejection Fraction, VT more likely still
Assess the ECG:
--P-waves in front of QRS? --Sinus
--Irregularly irregular? Atrial fib
(VT is regular, except for polymorphic VT which must have a polymorphic QRS)
--Regular? --then: sinus / atrial tach / flutter / PSVT / VT)
--Rate gradually changes or always the same?
Gradual: sinus or other automatic rhythm (some atrial tachycardias, junctional tach)
Unchanging: reentrant rhythm such as: flutter vs. PSVT (incl AVRT) vs. VT
Wide, monomorphic QRS, and regular without P-waves (or retrograde P-waves)
1. SVT with aberrancy
(including antidromic AVRT using an accessory pathway)
2. VT (though it may have P-waves that are dissociated, or retrograde P-waves)
VT vs. SVT with aberrancy
5 Algorithms to differentiate SVT with aberrancy from VT - in my opinion, only Sasaki's is usable in the ED:
1. Brugada
2. Vereckei 1
3. Vereckei 2 (uses aVR only)
4. Sasaki (see below) (Sasaki K. Circulation 2009; 120:S671) had 86% sensitivity and 97% specificity among 107 cases of wide complex tachycardia. It has not been validated; this is important: Brugada's rule fared much better in the initial study than in subsequent validation studies.
5. New algorithm (Jastrzebski et al.): more complex but more accurate (full text link):
The ventricular tachycardia score: a novel approach to electrocardiographic diagnosis of ventricular tachycardia. In this very complex scoring system, derived in 512 proven cases of VT and 276 proven cases of SVT, the specificity for a score greater than or equal to 3 was 98%, but with a sensitivity of only 66%. This is clearly not something that can be easily done in the ED.
Summary
Below I have listed what I consider usable features of the algorithms.
Management
If you're not sure, but you are pretty sure it is not sinus tach
Sedate/Cardiovert (or Adenosine)
Adenosine if you suspect SVT:
---Older, with known absence of structural heart disease
---Young age, unless known heart disease
---QRS duration less than 140 ms
---No obvious signs of VT
concordance, fusion beats, AV dissociation
---Unequivocal rapid depolarization of the initial part of the QRS (e.g., normal LBBB or RBBB)
--safe in VT
--safe in WPW, if regular rhythm
--Unsafe in WPW with atrial fib (irregular, RR intervals less than 250 ms, polymorphic QRS)
--converts reciprocating tachycardia, whether orthodromic or antidromic
--these depend on the AV node for re-entrance
--converts one kind of fascicular VT (RV outflow tract VT)
1) Look for hidden p-waves before each QRS. Don't miss sinus rhythm. Use Lewis Leads.
2) QRS duration: VT usually (but not always) has a QRS duration of greater than 140 ms. A prominent exception is fascicular VT. The wider the QRS, the more likely it is to be VT.
3) Is there RBBB or LBBB morphology and is the initial part of that BBB narrow? Then it is very likely to be SVT.
4) Do a quick look for obvious fusion beats and AV dissociation. If found, then VT.
5) Do a quick look for concordance (in precordial leads, all QRS's in the same direction -- this is not the same as concordance of ST segments in LBBB). Concordance means there is no RS.
A concordant QRS in the precordial leads comes in two varieties:
1) Upright QRS in V1 (RBBB type).
This is almost always VT or AVRT (antidromic, lateral accessory pathway)
2) Negative QRS in V1 (LBBB type).
This is VT at least 90% of the time, but not 100%.
6) Finally, because it is easy to apply, I like Sasaki's rule
Sasaki's rule
Step 1: Initial R in aVR?
This means is there a large single (upright) R-wave (not a small r-wave) in aVR. This indicates that the beats originate and propagate from the apex to the base, so that it must be coming from the ventricle, hence VT.
--If yes, then rhythm is VT. If no, step 2.
Not here. See ECG with line below. Although at first glance there appears to be a monophasic initial R in aVR, the line shows that there is actually a 30 ms delay.
Step 2: In any precordial lead, is the interval from onset of R-wave to the nadir of the S ≥ 100 msec (0.10 sec)? See image below.
--If yes, then rhythm is VT. If no, step 3. Not here.
Step 3: Initial r or q ≥ 40 ms in any lead?
If there is, this means that, for the first 40 or more milliseconds, conduction is slow as would occur through myocardium (left ventricle, VT), not through conducting fibers, as would occur in SVT)
--If yes, then it is VT. If no, then it is SVT. "No" here, therefore it is SVT
 |
Although there appears to be an initial R in aVR, there is actually about 30 ms of hesitation before the R-wave initiates. I have drawn a line at the beginning of the QRS.
Thus, there is also only a 30 ms r- or q- wave at the beginning of the QRS. |
This Sasaki rule was quite accurate in derivation, but never validated, at least not to my knowledge.
My interpretation is that the initial deflections are of very short duration and therefore unlikely to be VT. But I only saw it after knowing the outcome (too biased!)
So I showed the ECG blindly to
Ken Grauer (one of the masters, here is his site):
http://ecg-interpretation.blogspot.com/
"Bizarre morphology, which of course makes one consider VT —
but:
1) check leads (all positive in aVR); and
2) Could very well be
supraventricular with some severe form of underlying heart disease
(cardiomyopathy; congenital heart disease if young)"
"So I'd really want to
know the clinical situation....S
everal leads looked like they might be supraventricular (ie, very slender narrow initial r-wave in predominantly negative lead I and aVL — rather than being all negative) — slender initial r-wave in V4 with very steep downslope — and the Q in lead III looked like it may be pathologic rather than an all neg axis Q as VT would have."