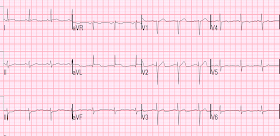
An elderly woman awoke with bilateral upper chest pressure and SOB. She called 911. Here is the first prehospital ECG:
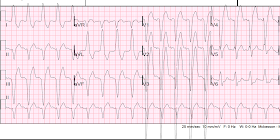
Here is the second prehospital ECG:
No single finding here is diagnostic of MI, but the whole pattern is: an elderly patient with typical ongoing pain, subtle STE with reciprocal STD, and 2 suggestive PVCs.
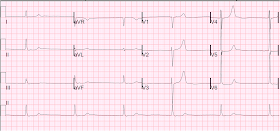
On arrival, pain persisted and an ED ECG was recorded:
I saw this patient and activated the cath lab immediately.
Angiography showed a large OM with 99% thrombotic occlusion in its proximal segment.
But it also showed: a mid LAD lesion with a 99% occlusion in its mid segment. (not noted whether this had acute thrombus)
The LAD lesion was a surprise to me, and whether it was a co-culprit is not noted. The large T-waves in V3 and V4 could be de Winter's waves, rather than posterior STEMI. These two entities may be confused.
Both lesions were stented.
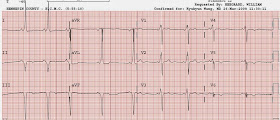
Here is the post cath ECG:
The troponin I peaked at 16.4 mEq/L
The post cath echo showed:
Normal LV size, mild concentric LV hypertrophy and severe systolic dysfunction, EF 20%.
Regional wall motion abnormality-distal septum anterior and apex, large and akinetic .
Regional wall motion abnormality-anterolateral, akinetic .
Regional wall motion abnormality-inferolateral, hypokinetic.
Regional wall motion abnormality-distal inferior, hypokinetic.
A study 2 days later was slightly improved, and one 3 days after the first had the following:
Contrast echo study: "MUCH OF THE RESTING RWMA NOTED YESTERDAY APPEARS TO HAVE NORMAL PERFUSION, IMPLYING POTENTIAL RECOVERY OF FUNCTION"
Which turned out to be true, as a 3 month convalescent echo showed:
The estimated left ventricular ejection fraction is 54%
The estimated pulmonary artery systolic pressure is 26 mmHg + RA pressure.
Normal estimated left ventricular ejection fraction lower limits of normal.
No wall motion abnormality
The patient did well.
Lessons:
1. Look at PVCs for evidence of ischemia. Here are many more such (great!) cases
2. Frequently, one finding on the ECG is not diagnostic, but the combination of several, which match ischemia of one or more coronary distributions, will make the diagnosis.
Here is the second prehospital ECG:
 |
| There is a PVC in V4-V6. The PVC in V5 has 2 mm STE after an 8 mm S-wave, for a ratio of 0.25. This would also be a high ratio for LBBB, but is it for a PVC? |
No single finding here is diagnostic of MI, but the whole pattern is: an elderly patient with typical ongoing pain, subtle STE with reciprocal STD, and 2 suggestive PVCs.
On arrival, pain persisted and an ED ECG was recorded:
 |
| Now there is reciprocal STD also in aVF, and new ST depression in V2 and V3, diagnostic of MI, almost certainly posterolateral. |
I saw this patient and activated the cath lab immediately.
Angiography showed a large OM with 99% thrombotic occlusion in its proximal segment.
But it also showed: a mid LAD lesion with a 99% occlusion in its mid segment. (not noted whether this had acute thrombus)
The LAD lesion was a surprise to me, and whether it was a co-culprit is not noted. The large T-waves in V3 and V4 could be de Winter's waves, rather than posterior STEMI. These two entities may be confused.
Both lesions were stented.
Here is the post cath ECG:
 |
| The ST depression is gone. T-waves in V3-V6 are all flattened now. |
The troponin I peaked at 16.4 mEq/L
The post cath echo showed:
Normal LV size, mild concentric LV hypertrophy and severe systolic dysfunction, EF 20%.
Regional wall motion abnormality-distal septum anterior and apex, large and akinetic .
Regional wall motion abnormality-anterolateral, akinetic .
Regional wall motion abnormality-inferolateral, hypokinetic.
Regional wall motion abnormality-distal inferior, hypokinetic.
A study 2 days later was slightly improved, and one 3 days after the first had the following:
Contrast echo study: "MUCH OF THE RESTING RWMA NOTED YESTERDAY APPEARS TO HAVE NORMAL PERFUSION, IMPLYING POTENTIAL RECOVERY OF FUNCTION"
Which turned out to be true, as a 3 month convalescent echo showed:
The estimated left ventricular ejection fraction is 54%
The estimated pulmonary artery systolic pressure is 26 mmHg + RA pressure.
Normal estimated left ventricular ejection fraction lower limits of normal.
No wall motion abnormality
The patient did well.
Lessons:
1. Look at PVCs for evidence of ischemia. Here are many more such (great!) cases
2. Frequently, one finding on the ECG is not diagnostic, but the combination of several, which match ischemia of one or more coronary distributions, will make the diagnosis.

















+anterior+STE+-+cropped.jpg)





.png)