This case was provided by Spencer Schwartz, an outstanding paramedic at Hennepin EMS who is on Hennepin EMS's specialized "P3" team, a team that receives extra training in advanced procedures such as RSI, thoracostomy, vasopressors, and prehospital ultrasound.
This patient, who is a mid 60s female with a history of hypertension, hyperlipidemia and GERD, called 911 because of chest pain. The fire department, who operate at an EMT level in this municipality, arrived before us and administered 324 mg of baby aspirin to the patient due to concern for ACS.
A mid 60s woman with history of hypertension, hyperlipidemia, and GERD called 911 for chest pain. On medic arrival, she walked out of the house in no distress, but was diaphoretic. She described intermittent chest discomfort for a week, and went to the clinic one day prior where a 12-lead was recorded and reportedly "normal." Today's pain felt similar to previous episodes of "Reflux;" it radiated to her neck and jaw.
VS were: BP 188/72, HR 88 and 99% SPO2 on room air.
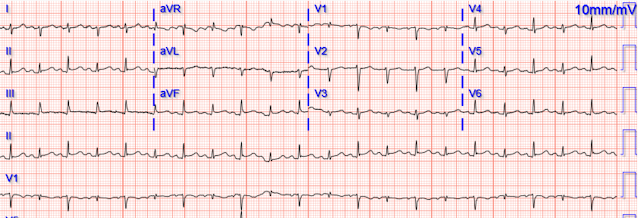
Our first 12 lead ECG was then recorded at 0926.
What do you think?
Here is Spencer's interpretation:
There is a sinus rhythm with narrow QRS complexes and a normal axis. V1 has 0.5 mm of ST segment elevation, V2 and V3 have 1 mm of elevation, v4 has 2 mm of elevation and v5 around 1.5 mm of elevation. More notably there are hyperacute T waves in V3 through V5. These T waves are too large in proportion to their QRS complexes with broad bases, and they are symmetric or nearly symmetric in appearance.There is also T wave inversion In aVL with a very small amount of ST segment depression. The T waves in the inferior leads appear to be turning hyperacute with broad bases and proportionally large size in comparison to their QRS complexes. The inferior T waves also appear to have a quick take off from the J point with a near symmetrical appearance.
These findings are suggestive of Occlusion of a wraparound LAD.
Smith: I would be more emphatic. These findings are diagnostic of an apical OMI as a result of LAD Occlusion.
Another ECG was recorded 5 minutes later just before arrival at the hospital:
The patient was transported to a nearby suburban hospital with PCI capabilities while my partner cared for her.
Upon arrival to the emergency department, a senior emergency physician looked at the ECG and said "Nothing too exciting."
Then she began complaining of severe dizziness and quickly went into ventricular fibrillation and resuscitation was initiated by hospital staff. She was defibrillated and resuscitated.
It is apparently fortunate that she had a cardiac arrest; otherwise, her ECG would have been ignored.
Smith: this ECG and clinical presentation is diagnostic of LAD Occlusion. I need to innoculate you against the subsequent opinions below. by making it clear to everyone that this is NOT an EKG that one sees with takotsubo cardiomyopathy. It is also NOT the clinical scenario of takotsubo (a week of intermittent chest pain). Takotsubo is a sudden event, not one with crescendo angina. An apical OMI has the same ultrasound findings as takotsubo, and thus mimics takotsubo.
Hospital Course
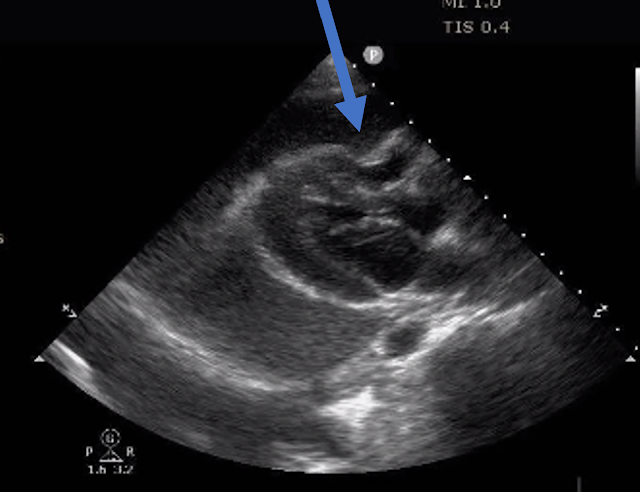
The patient was taken emergently to the cath lab which did not reveal any significant coronary artery disease, but she was noted to have reduced EF consistent with Takotsubo cardiomyopathy.Takotsubo Cardiomyopathy (EF 30-35%)
V Fib Cardiac arrest
Prolonged QTC
NSTEMI (Smith comment: is it NSTEMI or is it Takotsubo? -- these are entirely different)
Moderate single-vessel CAD.
Such cases are classified as MINOCA (Myocardial Infarction with Non-Obstructed Coronary Arteries). MINOCA has many etiologies. One of the most common is rupture of a non-obstructive plaque, with thrombus formation and OMI that spontaneously lyses and leaves a wide open artery. We can tell from the history and ECG that this case is MINOCA that was a result of transient LAD Occlusion with thrombus that subsequently lysed. One need not have obstructive coronary disease to have occlusive thrombus! In fact, the majority of acute MI occur in coronary arteries that do not have hemodynamically significant stenoses (see New England Journal Review below)
Pathogenesis of Acute Coronary Syndromes
Findings from clinical and pathological studies have challenged these commonly held notions of the pathophysiological features of coronary atherosclerosis and its treatment.1-4 Surprisingly, serial angiographic studies have revealed that the plaque at the site of the culprit lesion of a future acute myocardial infarction often does not cause stenosis that, as seen on the antecedent angiogram, is sufficiently severe to limit flow. Angiographic monitoring of responses to thrombolytic therapy has shown that after lysis of the offending thrombus, the underlying stenosis is often not the cause of the critical stenosis of the artery. In a prospective angiographic study involving patients undergoing percutaneous intervention for coronary artery disease, only half the subsequent events arose from lesions with sufficient stenosis to have warranted intervention at the time of revascularization.5 Computed tomographic (CT) angiography, which permits evaluation of the arterial wall (not just the lumen), has shown that the characteristics of plaque associated with acute coronary syndromes include low attenuation (i.e., little or no calcification) and outward expansion of the artery wall, a process that tends to accommodate the growth of plaque while minimizing luminal encroachment.6-8 Intravascular ultrasonography has shown that in acute coronary syndromes, the culprits often lie proximal to the sites of maximal stenosis — the traditional targets of revascularization therapies.9 This dissociation between the degree of stenosis and the propensity to provoke an acute coronary syndrome helps to explain why myocardial infarction often occurs without being heralded by the demand-induced symptoms of angina that would result from a high-grade stenosis.
Acute thrombosis at the site of non-obstructive eccentric plaque thrombosis — Many atherosclerotic plaques expand outward rather than encroaching on the arterial lumen. These ”positively-remodelled” plaques are often lipid rich and have a thin fibrous cap; they are vulnerable to rupture into the lumen [1,9,10]. Transient and partial thrombosis at the site of a non-obstructive plaque with subsequent spontaneous fibrinolysis and distal embolization may be one of the mechanisms responsible for the occurrence of MINOCA. Similarly, coronary erosion with loss of surface endothelium, possibly due to hyaluronan and neutrophil accumulation, can also cause MINOCA [1,11]. (See "Mechanisms of acute coronary syndromes related to atherosclerosis".)
The reason for these cases to be labeled as MINOCA is that angiography is of limited utility for the purpose of elucidating plaque-related thrombosis as a cause of thrombosis due to its low resolution as well as the fact that it does not interrogate the lumen of the vessel. Thus, intracoronary imaging modalities are crucial in this setting. Plaque rupture or erosion has been diagnosed by intravascular ultrasound in about 40 percent of women with MINOCA [12]. Optical coherence tomography, due to its high resolution, may provide additional information [10,13].
As MINOCA is associated with a risk of recurrent cardiovascular events over time, comparable with that of patients with acute coronary syndromes (ACS) and obstructive atherosclerosis [5,14,15], these patients require dual antiplatelet treatment for 12 months and statins. In particular, long-term lipid-lowering therapy with statins after MI is associated with a significant increase of the fibrous-cap thickness, paralleling the reduction of the lipid content of the plaque [16]. (See "Prevention of cardiovascular disease events in those with established disease (secondary prevention) or at very high risk".)
From Gue at al.
STEMI MINOCA versus NSTEMI MINOCA
STEMI occurs in the presence of transmural ischaemia due to transient or persistent complete occlusion of the infarct-related coronary artery. In patients presenting with non-ST-segment elevation MI (NSTEMI), the infarct is subendocardial. This pathophysiological difference also seems to be present within the MINOCA cohort. Registry data indicate that 6–11% of patients with acute MI have nonobstructive coronary arteries. Within the literature, MINOCA tends to present more commonly as NSTEMI than STEMI: the incidence of MINOCA reported in patients presenting with NSTEMI is about 8–10% and in STEMI cohorts it is 2.8–4.4%. This has resulted in an under-representation of STEMI MINOCA patients in the literature. Most studies examine undifferentiated ACS cohorts, with only a handful providing separate data. These studies indicate that the 1-year mortality of MINOCA presenting as STEMI is 4.5%, in contrast to the mortality of unselected MINOCA ACS patients who have a mortality of 4.7%. The underlying aetiology of MINOCA is similar among those presenting with STEMI and in all-comer MINOCA patients with ACS, with non-coronary aetiology responsible for presentation in 60–70% of individuals with STEMI and in 76% of unselected ACS patients.
References:
1. Lindahl B, Baron T, Erlinge D, et al. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation [Internet] 2017;135(16):1481–9. Available from: http://dx.doi.org/10.1161/CIRCULATIONAHA.116.026336 https://www.ahajournals.org/doi/epdf/10.1161/CIRCULATIONAHA.116.026336
2. Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): The Past, Present, and Future Management [Internet]. Circulation. 2017;135(16):1490–3. Available from: http://dx.doi.org/10.1161/CIRCULATIONAHA.117.027666 https://www.ahajournals.org/doi/epdf/10.1161/CIRCULATIONAHA.117.027666
3. Gue YX, Kanji R, Gati S, Gorog DA. MI with Non-obstructive Coronary Artery Presenting with STEMI: A Review of Incidence, Aetiology, Assessment and Treatment. Eur Cardiol [Internet] 2020;15:e20. Available from: http://dx.doi.org/10.15420/ecr.2019.13
4. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med [Internet] 2013;368(21):2004–13. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23697515
- My focus today is on brief additional commentary regarding the ECGs in this case — and — some thoughts about MINOCA (MI with Non-Obstructive Coronary Arteries).
- Even more leads than were mentioned show acute changes in today's case! While this point is not essential for initial management (ie, Spencer emphasized his suspicion for acute OMI — with need for transport to a facility with PCI capability) — there are plenty of cases in which recognition of how many leads actually show acute changes is important for accurate diagnosis.
- As per Spencer — the most notable changes in ECG #1 — are the hyperacute ST-T waves in anterior leads V3-thru-V5.
- Regarding the chest leads: By the concept of "neighboring leads" — the T waves in leads V2 and V6 are also hyperacute. By this I mean that in isolation — the T waves in leads V2 and V6 might not seem abnormal. But both of these leads do show a T wave that is "fatter"-at-its-peak and wider-at-its-base than it should be — so in the context of obviously hyperacute ST-T waves in leads V3-V5 — I interpreted the range of acute ST-T wave changes as encompassing leads V2-thru-V6.
- In addition — the deep and wide Q waves in leads V1,V2 (with no more than the tiniest of initial r wave in lead V3) — suggests significant myocardial injury has already occurred in the anterior myocardium.
- Regarding the limb leads: More than just "turning" hyperacute — the ST-T waves in each of the inferior leads are hyperacute for the very reasons that Spencer mentions ( = these inferior T waves are too large in proportion to their QRS complexes — with broad bases) — as well as also being "fatter"-than-they-should-be at their peak. My point being simply that while I might not be certain from the inferior lead ST-T wave appearance alone that there are acute changes — I know that there are acute inferior lead changes in the context of the obviously hyperacute chest lead changes (supported as per Spencer — by reciprocal T wave inversion in lead aVL).
- Spencer correctly identified key abnormalities while expediting transport to a PCI-capable facility. But as per Dr. Smith — it is worth emphasizing that in a patient who presents with new chest pain (as in today's case) — the findings in ECG #1 are truly diagnostic of acute LAD OMI until you prove otherwise.
- To Emphasize: The reason definitive diagnosis is important in today's case — is that the senior ED physician interpreted ECG #1 as "nothing too exciting". It is essential to impress upon that physician that we are not dealing with a "maybe" — but rather with an ECG that provides definitive diagnosis of acute LAD OMI in need of immediate cath until proven otherwise.
- Beyond-The-Core: As discussed in the November 13, 2022 post by Emre Aslanger in Dr. Smith's ECG Blog — ST elevation in both anterior and inferior leads does not necessarily indicate an LAD "wraparound" lesion, as used to be thought (Bozbeyoğlu, Yildirimtürk, Aslanger et al — Anatol J Cardiol 21:253-8, 2019).
- As per Dr. Smith — the cardiology opinion following evaluative testing on today's patient was contradictory — because "NSTEMI" is a very different entity from Takotsubo Cardiomyopathy.
- PEARL #1: As we have often emphasized on Dr. Smith's ECG Blog — Because of the common (albeit still not well appreciated) phenomenon of spontaneous reperfusion of the "culprit" coronary artery (which may sometimes reopen and reocclude multiple times) — more than a single ECG is often needed to identify a recent OMI that shows a relatively normal initial tracing as a result of the "pseudonormalization" that may be seen when acute chest pain resolves because the "culprit" artery has spontaneously reperfused. In order to fully appreciate the sequence of events — serial timed ECGs that are all correlated to serial timed troponin assays and chest pain severity scores may be needed.
- As per Dr. Smith — the post-reperfusion ECG seen in Figure-1 is not consistent with the expected findings of Takotsubo Cardiomyopathy. (For review of ECG findings expected with Takotsubo Cardiomyopathy — Please see My Comment at the bottom of the page in the March 25, 2020 post in Dr. Smith's ECG Blog). Instead — We see typical reperfusion T waves in the diffuse distribution of what was hyperacute ST-T wave changes on the initial tracing.
- The "good news" — is that anterior R wave progression has improved since the initial tracing (ie, There are now definite initial R waves in leads V2 and V3 of ECG #3 — without loss of R wave amplitude in other leads).
-USE.png) |
| Figure-1: Comparison of the initial ECG in today's case — with the post-reperfusion ECG. (To improve visualization — I've digitized the original ECG using PMcardio). |
- As per Dr. Smith — Perhaps the most common etiology of MINOCA is plaque rupture with thrombus formation and OMI that spontaneously lyses, resulting in a patent "culprit" artery at the time of cardiac catheterization.
- Putting Today's Case Together: The history of intermittent chest discomfort over the course of 1 week — together with the initial and post-reperfusion ECG findings — is most consistent with acute apical OMI. This impression was confirmed on cardiac MRI.
- PEARL #2: The fact that the initial ECG in today's case showed hyperacute changes in at least 9 leads in the infero-antero-lateral distribution is consistent with acute apical OMI. As per Dr. Smith — it's important to appreciate that Echo findings of an extensive apical OMI may mimic those of Takotsubo Cardiomyopathy!
- PEARL #3: The importance of Figure-2 — is that it highlights the need to open ourselves to a series of diagnostic entities when confronted with a patient showing positive troponins and an ECG suggestive of acute MI despite the finding of non-obstructive coronary disease on cath.
-USE.png) |
| Figure-2: Classification of Underlying Diagnoses in Patients with MINOCA (Adapted from Table-1 in Sykes et al: Interventional Cardiology Review: 16:e10, 2021). NOTE: As per Sykes et al — The entities listed under "Other Etiology" may be diagnosed following further investigation and should be considered separately (because they are typically associated with myocardial injury but not considered an MI by the 4th universal definition of MI). This is an important indication for cardiac MRI in patients suspected of MINOCA. |







-USE.png)

-USE.png)