Written by Pendell Meyers, submitted by Daryl Williams, edits by Steve Smith
A man in his sixties with prior CAD and CABG experienced chest pain and pressure off and on for three days. He saw his primary doctor during this time who had suspected GI related symptoms and increased his PPI medication. On the third day it became more intense and had associated radiation to his neck and left arm, and this reminded the patient of his prior MI symptoms, so he presented to the Emergency Department. It is unclear how long he had constant symptoms during those three days.
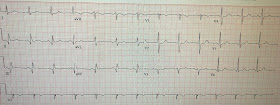
Here is his triage ECG (no prior was available in our system):
Raw Findings:
- Normal, narrow QRS complex (other than the Q waves)
- 0.5 mm STE in III and aVF
- STD in I and aVL, V2-V5 (maximal in V2)
- Q waves in II, III, and aVF, as well as very small Q waves in V5 and V6
Interpretation:
Acute to subacute inferoposterior OMI
Inferior STE with Q waves and slight STE without obviously hyperacute T waves could be acute, subacute, chronic (inferior LV aneurysm), or acute on chronic (new MI superimposed on an old LV aneurysm.
The tall R-wave in V2 could be due to old or subacute posterior MI.
Old MI with persistent STE, just like acute MI, may indeed have reciprocal ST depression (aVL) and old posterior MI (posterior aneurysm) can have persistent ST depression. So a large R-wave in V2 with ST depression could be old or subacute, as well as acute.
There is no good way to differentiate inferior OMI from inferior aneurysm. Both may have QR-waves and absence of large T-waves. This is in contrast to anterior LV aneurysm, which is distinguished from acute anterior OMI by absence of hyperacute T-waves (if your differential dx is anterior OMI vs. LV aneurysm, and the chest pain duration is less than 6 hours, then if there is one lead of V1-V4 that has a T/QRS ratio of greater than 0.36, then it is most likely acute.
Thus, in this case with ongoing symptoms, we must assume these ECG findings are acute or subacute. Of course, this ECG does not meet STEMI criteria.
As always, you must remember that the ECG does not differentiate between etiologies of acute transmural injury, it simply reports those conditions experienced by the myocardial cells. The provider must decide clinically what do to with this differential, including OMI, takotsubo cardiomyopathy, focal myocarditis, etc. However, OMI is overwhelmingly the most common and treatable etiology in the differential, the most likely etiology for this ECG alone, and overwhelmingly most likely given the history we have for the patient. Furthermore, the other diagnoses are basically diagnoses of exclusion to be made only after a negative angiogram. The importance of this understanding is that you will be prepared for those rare times when the cath is completely negative, and you will understand that the decision to perform the cath was still the right decision.
The team immediately recognized the concerning history and the subtle but definite findings on the ECG and called the interventionalist to the bedside for emergent cath evaluation.
During that time the team decided to record posterior and right sided leads in case they might help persuade the interventionalist to recognize the findings on the standard 12 lead.
This ECG is labelled as posterior leads in the records, but is is unclear whether this is truly posterior:
The patient did well.
Learning Points:
Although we frequently explain on this blog that the posterior and R sided leads are unlikely to add important information to expert interpretation of the standard 12-lead, we do acknowledge that they can add to subtle findings, revealing injury patterns that might otherwise go unnoticed. This was the case here, with subtle standard 12-lead findings, but with slightly more obvious STE in the R sided leads.
If guidelines actually recognize posterior and R sided leads in some version of the STEMI criteria, then they only require 0.5 mm in a single lead.
Ongoing pain and/or ongoing ECG evidence of ischemia should be interpreted as ongoing myocardial loss (and therefore ongoing opportunity to salvage myocardium), regardless of the timing of patient symptoms or Q waves on ECG, especially when you have the option to perform PCI.
A man in his sixties with prior CAD and CABG experienced chest pain and pressure off and on for three days. He saw his primary doctor during this time who had suspected GI related symptoms and increased his PPI medication. On the third day it became more intense and had associated radiation to his neck and left arm, and this reminded the patient of his prior MI symptoms, so he presented to the Emergency Department. It is unclear how long he had constant symptoms during those three days.
Here is his triage ECG (no prior was available in our system):
 |
| What do you think? |
Raw Findings:
- Normal, narrow QRS complex (other than the Q waves)
- 0.5 mm STE in III and aVF
- STD in I and aVL, V2-V5 (maximal in V2)
- Q waves in II, III, and aVF, as well as very small Q waves in V5 and V6
Interpretation:
Acute to subacute inferoposterior OMI
Inferior STE with Q waves and slight STE without obviously hyperacute T waves could be acute, subacute, chronic (inferior LV aneurysm), or acute on chronic (new MI superimposed on an old LV aneurysm.
The tall R-wave in V2 could be due to old or subacute posterior MI.
Old MI with persistent STE, just like acute MI, may indeed have reciprocal ST depression (aVL) and old posterior MI (posterior aneurysm) can have persistent ST depression. So a large R-wave in V2 with ST depression could be old or subacute, as well as acute.
There is no good way to differentiate inferior OMI from inferior aneurysm. Both may have QR-waves and absence of large T-waves. This is in contrast to anterior LV aneurysm, which is distinguished from acute anterior OMI by absence of hyperacute T-waves (if your differential dx is anterior OMI vs. LV aneurysm, and the chest pain duration is less than 6 hours, then if there is one lead of V1-V4 that has a T/QRS ratio of greater than 0.36, then it is most likely acute.
Thus, in this case with ongoing symptoms, we must assume these ECG findings are acute or subacute. Of course, this ECG does not meet STEMI criteria.
As always, you must remember that the ECG does not differentiate between etiologies of acute transmural injury, it simply reports those conditions experienced by the myocardial cells. The provider must decide clinically what do to with this differential, including OMI, takotsubo cardiomyopathy, focal myocarditis, etc. However, OMI is overwhelmingly the most common and treatable etiology in the differential, the most likely etiology for this ECG alone, and overwhelmingly most likely given the history we have for the patient. Furthermore, the other diagnoses are basically diagnoses of exclusion to be made only after a negative angiogram. The importance of this understanding is that you will be prepared for those rare times when the cath is completely negative, and you will understand that the decision to perform the cath was still the right decision.
The team immediately recognized the concerning history and the subtle but definite findings on the ECG and called the interventionalist to the bedside for emergent cath evaluation.
During that time the team decided to record posterior and right sided leads in case they might help persuade the interventionalist to recognize the findings on the standard 12 lead.
This ECG is labelled as posterior leads in the records, but is is unclear whether this is truly posterior:
The team reported that the posterior leads did not show any STE whatsoever, and next tried right sided leads (V3-V6 are actually V3R-V6R, V1 and V2 remain the same):
 |
| There is between 0.5 and 1.0 mm of STE in leads V5 and V6, with Q waves. |
Here are the right sided leads enlarged:
Although it remains unclear whether the ACC/AHA guidelines actually, formally recognize any STEMI equivalent criteria in the posterior and/or right sided leads, there is a belief among some providers that they do so. The standard (or proposed) cutoff is 0.5 mm in a single right sided or posterior lead.
After recording this posterior ECG, and having had time to review the patient's high risk history and clinical symptoms, the cardiologist agreed that emergent cath was indicated.
At cath, they found that his saphenous vein bypass graft to the RCA had 100% acute thrombotic occlusion, and was unable to be crossed with the wire, thus no intervention was possible. He also received a stent to OM1 with 99% stenosis, but it was not clear that this lesion was an acute culprit.
Does the angiogram explain these findings entirely?
For both of these potential lesions, it seems somewhat unusual to have maximal findings in the rightward leads, and we are not totally sure if either of these lesions fully explains the ECG findings. Typically the RV marginal branch supplies the RV free wall and produces findings most localized to the right sided leads. The RV marginal branch comes from the proximal RCA, soon after the ostium, and thus is usually involved with very proximal or ostial RCA occlusions. This patient had a chronic mid-RCA occlusion, with a distal RCA bypass, which suggests that the proximal RCA was still patent. This makes it somewhat confusing that a distal RCA bypass occlusion would involve territory thought to be supplied by the RV marginal branch, but there are many anatomic variants and ultimately this is not the most important part of clinical practice.
The first troponin T resulted at 0.36 ng/mL. The second troponin was 0.27, and no more troponins were measured after that.
Here is his ECG the next morning:
The patient did well.
Learning Points:
Although we frequently explain on this blog that the posterior and R sided leads are unlikely to add important information to expert interpretation of the standard 12-lead, we do acknowledge that they can add to subtle findings, revealing injury patterns that might otherwise go unnoticed. This was the case here, with subtle standard 12-lead findings, but with slightly more obvious STE in the R sided leads.
If guidelines actually recognize posterior and R sided leads in some version of the STEMI criteria, then they only require 0.5 mm in a single lead.
Ongoing pain and/or ongoing ECG evidence of ischemia should be interpreted as ongoing myocardial loss (and therefore ongoing opportunity to salvage myocardium), regardless of the timing of patient symptoms or Q waves on ECG, especially when you have the option to perform PCI.
===================================
MY Comment by KEN GRAUER, MD (7/19/2020):
===================================
Insightful case by Drs. Williams, Meyers & Smith — that highlights how selective use of right-sided leads can be extremely helpful when the ECG picture from the standard 12-lead tracing is equivocal.
- The patient in today’s case was a 60-something man with known coronary disease, including prior CABG. He presented to the ED with intermittent symptoms of chest discomfort over the preceding 3 days — that became more constant and severe on the day of admission.
- I’ve numbered the tracings shown above in the sequence presented — and limit my comments to 3 tracings, that I’ve put together for clarity in Figure-1.
QUESTIONS: Dr. Meyers has noted the principal ECG findings in his discussion above. For the purpose of honing in on some ECG interpretation fine points — I’ll rhetorically ask the following questions:
- In ECG #1 — WHAT should YOU be thinking as you contemplate the sequential ECG appearance of leads V1, V2 and V3? What could (should have been) done? — since the goal of the team was “to help persuade the interventionalist” to perform emergent cath?
- How GOOD is the standard 12-lead ECG for predicting acute RV infarction? Are any of the signs we look for present in ECG-1?
- In ECG #3 — WHICH of the right-sided leads are abnormal?
- Compared to ECG #1 — HOW MANY of the 12 leads in ECG #5 show relevant changes? HINT: If your answer is less than 9 — then Count again!
 |
| Figure-1: The 1st, 3rd and last tracing shown above in today’s case (See text). |
My THOUGHTS Regarding Today’s Case: I’ll emphasize at the outset that many of the points I highlight below are subtle and advanced — but all are relevant to today’s case:
- Looking first at ECG #1: The appearance of the QRST complex in lead V2 struck me as bizarre — given the appearance of neighboring leads V1 and V3. It does not make physiologic sense to me how it is possible to go from a 4-component (rSr’s’) complex of small amplitude in lead V1 — to what we see in lead V2 — followed immediately by another very small amplitude complex with tiny r wave and reduced T wave amplitude in lead V3 — which is then immediately followed by abrupt transition change again occurring in lead V4. The most logical explanation for this highly unusual sequence of events is that there must be inappropriate positioning of one or more of the anterior chest leads. Of Note: The ECG done the next morning ( = ECG #5) — shows a much more logical transition sequence of QRST appearance as one moves from lead V1-to-V2-to-V3-to-V4, V5 and V6. This supports my presumption that there had to have been faulty lead placement of one or more anterior chest lead electrodes in ECG #1.
- Learning Point: Since the ED team correctly suspected that they might have a hard time “persuading” the interventionalist to do emergent cath — it may have been helpful to immediately repeat ECG #1 while verifying correct lead placement on this repeat ECG. Recognizing inappropriate chest lead placement — and, immediately repeating the ECG when searching for clues to an acute evolving process is likely to have a higher probability of success in this case than obtaining posterior leads.
- Posterior Leads are not sensitive for detecting acute posterior infarction. I’ll emphasize that I am not saying you should never do posterior leads — but rather, that with a little practice — it is possible to make the diagnosis of acute posterior OMI much more quickly, simply by using the standard 12-lead ECG (ie, by considering the mirror-image appearance of anterior leads V1, V2 and/or V3). Thus, the unexpectedly tall R wave + shelf-like (flat) ST depression with unexpectedly tall terminal T wave in lead V2 of ECG #1 tells you that there has been posterior infarction, albeit of uncertain age.
- Clinically — given lack of a prior ECG for comparison in this 60-something man with known coronary disease, who now presents with increasing new chest pain — the “Answer” (ie, the need for prompt diagnostic/therapeutic cath) is already evident from the standard 12-lead in ECG #1. I don’t think I’ve seen a case in which posterior leads told me something that I did not already know from use of the standard 12-lead ECG (For more on use of the Mirror Test for ECG assessment of acute posterior MI — SEE My Comment at the bottom of the February 16, 2019 post in Dr. Smith’s ECG Blog).
Use of the Standard 12-Lead ECG for Detecting Acute RV MI: As opposed to posterior leads, that provide limited benefit (at most) for detecting acute posterior MI when this diagnosis is not already evident from the standard 12-leads — Right-Sided Leads may prove invaluable in suggesting acute RV MI that was not evident from the standard 12 lead ECG.
- Acute inferior MI is associated with acute RV involvement in more than 1/4 of cases. This is clinically relevant — since the clinical course, prognosis and treatment of hemodynamically significant acute RV MI differ markedly from that of acute LV MI (ie, hypotension, relative hypovolemia, hypersensitivity to nitroglycerin, etc. with acute RV involvement).
- Approximately 80-90% of people have a right-dominant coronary circulation — in which the RCA (Right Coronary Artery) is a dominant vessel that supplies the RV early in its course — and then continues (after supplying the RV) as the PDA (Posterior Descending Artery) along the undersurface of the heart to supply the posterior and inferior walls of the LV.
- In about 15% of people — the LCx (Left Circumflex) is a dominant artery — in which case, the RCA is a much smaller vessel — and, the LCx gives rise to the PDA, thereby supplying the inferior and posterior walls, as well as the lateral wall of the LV. (CLICK HERE — For more on the Coronary Circulation).
In patients with acute inferior MI — ECG Predictors of acute RCA occlusion (rather than dominant LCx occlusion) include: i) ST elevation in lead III > II; ii) Marked ST depression in lead aVL; iii) If there is ST elevation in leads V5 and V6 — it is less than the amount of ST elevation in lead III; and, iv) There is ECG evidence of acute RV MI.
- PEARL #1 — This last predictor (iv) in the above bullet is the “theme” of today’s case — since we can confirm the RCA as the “culprit” artery with acute inferior infarction IF there is also ECG evidence of acute RV MI. This is because the LCx does not supply the RV. And, because the RV is supplied by the RCA early in its course — ECG evidence of acute RV MI tells you there is a proximal RCA occlusion.
- Because none of the standard 12 ECG leads directly view the RV — the sensitivity of the ECG for detecting acute RV MI is not optimal. This means that much of the time — you will not be able to rule out the possibility of acute RV MI from exclusive use of the standard 12 leads. That said — lead V1 comes closest to viewing electrical activity in the right ventricle — so, as we’ve shown on numerous posts in Dr. Smith’s ECG Blog — IF there is abnormal ST elevation in lead V1 (but not in other anterior leads) in association with new-onset chest pain and acute inferior lead ST elevation — then the standard 12-lead ECG can be strongly suggestive of acute RV MI from acute proximal RCA occlusion. (For just one example of ECG diagnosis of acute RV MI from the standard 12 leads — Check out the October 7, 2019 post, with attention to My Comment at the bottom of the page).
- PEARL #2 — Right-sided chest lead ST elevation in association with acute RV MI tends to be transient! Right-sided ST elevation has been shown to resolve in up to 50% of patients with acute RV MI within 12 hours of the onset of symptoms! (Nagam et al — Perm. J 2017 ;21:16-105). This is relevant to today’s case — as the onset of symptoms in today’s patient was at least 3 days earlier! Perhaps this is the reason there was no ST elevation seen in lead V1 by the time ECG #1 was finally done?
Is there Any Suggestion of Acute RV MI in ECG #1? Although definitive ECG evidence of acute RV MI is clearly not present in ECG #1 — I thought this tracing is suggestive of this possibility!
- As per Dr. Meyers — ECG #1 is diagnostic of inferior MI of uncertain age. The deep and wide Q waves in each of the inferior leads tell us there has been inferior infarction at some point in time in this 60-something man who underwent prior CABG. But, in the absence of a prior ECG for comparison — there is NO way to know if the slight-but-real ST elevation in leads III and aVF is new or old. ST segment straightening of the takeoff in lead II of ECG #1 could be a hyperacute change — as could the ST segment straightening and hint of depression in lead aVL. As we’ve previously noted — the most marked ST-T wave changes in ECG #1 appear in lead V2 — but it is equally possible that all of these findings are old. BOTTOM Line: As per Dr. Meyers — we simply can not tell from ECG #1 alone if a new acute event is in progress.
- PEARL #3 — The above said, if this patient was evolving an acute event — then the shape of the ST segment in lead V1 is not normal (and this should make you suspicious of associated acute RV MI). Especially in the context of the unexpectedly straight (shelf-like) ST depression in lead V2 of ECG #1 — the coving (curved RED line in this tracing) of the ST segment in lead V1 (even though there is no ST elevation) is not what should normally be expected in association with the posterior infarction that we diagnosed from the tall R wave and ST-T wave appearance in lead V2 (For more on the “ECG interplay” in anterior leads in the setting of both posterior and RV infarction — SEE My Comment at the bottom of the October 7, 2019 post).
PEARL #4 — As per Dr. Meyers, right-sided leads are needed for definitive diagnosis of acute RV MI in today’s case (ECG #3). When precordial leads are placed on the right side of the chest — lead V2 becomes right-sided lead V1 ( = V1R) — and — lead V1 becomes right-sided lead V2 ( = V2R). Although some providers prefer leaving standard leads V1 and V2 unchanged — and recording leads V3R-thru-V6R when getting right-sided leads (as was done above for the 3rd ECG in Dr. Meyers discussion) — I prefer reversing chest lead placement of all 6 leads, as I’ve done in ECG #3, as this allows for logical lead-by-lead progression of the 6 right-sided leads. It is purely a matter of personal preference as to whether you switch leads V1 and V2 when recording right-sided leads!
- The BEST single right-sided lead to use when assessing for acute RV MI is lead V4R. When there is ≥1 mm of ST elevation in lead V4R — this approaches 100% sensitivity and over 90% predictive accuracy for detecting acute RV MI (Jeffers et al — StatPearls- Updated May, 2020).
- Other parameters that have been cited as predictive of acute RV MI include: i) ST elevation in any of the other right-sided leads; and, ii) Progressive increase in the relative amount of right-sided ST elevation as one moves rightward from lead V1R-to-lead V6R.
- In ECG #3 — there is ST elevation in 5 consecutive right-sided leads, beginning with lead V2R — and, right-sided ST elevation is maximal in leads V5R and V6R. These right-sided ECG findings confirm acute RV MI!
The final points to make regarding today’s case relate to comparison of ECG #5 (which was done the next morning) — with the initial ECG done in the ED ( = ECG #1). The ECG findings highlighted above by Dr. Meyers are easiest to observe when both of these tracings are placed together, as they are in Figure-1.
- The 1st thing to note (as I mentioned at the beginning of my Comment) — is that chest lead progression of the QRST complexes in leads V1-thru-V4 seems much more logical in ECG #5, compared to the bizarre sequential appearance for these leads in ECG #1. Since ECG #5 was done the day after ECG #1 (presumably on the hospital floor, and no longer in the ED) — chances are that a different ECG technician did this next day recording. As a result — comparison of anterior lead appearance between ECGs #1 and #5 may have been altered by a change in electrode lead placement ... (ie, I have no idea if the greatly reduced R wave amplitude in lead V2 of ECG #5, compared to R wave amplitude of this lead in ECG #1 is a real effect relating to posterior MI or not).
- What we can definitely say — is that despite no appreciable change in size or distribution of the large inferior Q waves, there is now subtle-but-real suggestion in ECG #5 of reperfusion ST-T wave changes in the inferior leads (ie, flattening of the hyperacute straight ST segment takeoff we saw in lead II of ECG #1 — and evolution into subtle-but-real T wave inversion in leads III and aVF). In addition, I believe the subtle-but-real increase in T wave amplitude in lead aVL of ECG #5 is a mirror-image reflection of the reperfusion T wave inversion we see in lead III.
- In the chest leads of ECG #5 — while difficult to comment on anterior lead changes (due to the probable error in electrode lead placement) — there has been obvious flattening of the ST-T waves in lateral chest leads V4, V5 and V6. I interpreted this as additional evidence of reperfusion ST-T wave changes.
Our THANKS to Drs. Meyers and Williams for the insights provided by this case!




Hello, great case.
ReplyDeleteI have a question for Dr. Grauer.
Why is the T wave in V2 considered unexpectedly tall?
I see it appear very different than V1 and V3 also broader and taller than the T waves in the other precordial leads. Is this the reason?
@ plqstich — Ideally, there is gradual progression of the R wave as you move across the chest leads, at least until lead V4 or V5. For example, in my Figure-5 — ECG #5 (Bottom tracing) has reasonable R wave progression. While the R wave is a little-bit-taller-than-expected in leaad V2 — and while “ideally” the R wave should not get smaller again (as it then does in lead V3) — this is not a marked difference. Following this we see clear transition in ECG #5 by lead V4, with normal R wave progression in V5 and V6. Usually R wave amplitude tails off after V4 or V5 (so the R in V6 is often a little less tall than in V5). There are MANY variations on this “theme” — and there are numerous causes of “poor” R wave progression — not all of which are due to pathology. But overall — I’d say R wave progression in ECG #5 is within an “expected” range.
DeleteWhat IS definitely different in ECG #1 (TOP tracing) — is that both the R wave AND the S wave in lead V2 become unexpectedly large compared to the much smaller QRS complex before it ( = V1) and after it ( = V3) — and this is NOT a “normal” R wave (or S wave) progression. It just looks “out of place”. The reason this is relevant in this case — is that we are looking for subtle changes in ST-T waves. Note how different the ST-T waves look in lead V1 of ECG #1 — vs the ST-T wave in V2 — vs in V3. WHICH ONE(s) is correct?
Now imagine in your mind that for this TOP ( = ECG #1) tracing — that lead V2 and V3 were reversed. THEN there would be a much more logical (thought not “perfect”) progression of both R wave, as well as ST-T wave. I don’t know if simple reversal of leads V2 or V3 is the problem … or if there is mistaken anatomical placement of one or more of these anterior leads — but I suspect something is wrong here. What we found when looking at electrode lead placement, is a disturbing number of even trained clinicians (and techs) making obvious errors in lead placement — and the problem (especially when different techs are assigned to do different ECGs on patients having serial tracings) — is that this may significantly alter the consistency of ST-T wave appearance (and therefore affect interpretation). THANKS again for your question! — :)
Thank you for the detailed explanation, it is clear to me now
Deletependell, daryl williams, steve and ken...
ReplyDeletethank you.
excellent example of impressive right heart lead STE.
thanks, all
tom
Our pleasure Tom — :)
Delete