An elderly woman had sudden ventricular fibrillation.
She was unable to be defibrillated but was cannulated and placed on ECMO in our Emergency Department (ECLS - extracorporeal life support). ECMO Flow was achieved after approximately 1 hour of high quality CPR.
After good ECMO flow was established, she was successfully defibrillated.
Here is her monitor rhythm:
She then had a 12-lead:
There is sinus bradycardia with one PVC. There is "Shark Fin morphology"
I saw this and thought for certain that this was going to be an LAD or left main occlusion as etiology of arrest, and etiology of profound ST Elevation in I, II, aVL, and V3-V6, and ST depression in III, V1 and V2.
The K was normal.
Here is a case of ECMO defibrillation with near shark fin that was due to proximal LAD occlusion.
Angiography showed normal coronaries.
The etiology of arrest was not determined.
Troponin I rose to 44.1 ng/mL (equivalent to an hs trop I of 44,000 ng/L). There were no further troponins, so we do not know the peak. This is a troponin I level that is almost exclusively seen in STEMI. In this case, profound shock for 1 hour would result in the same degree of infarction.
I suspect this is Type 2 MI due to prolonged severe hypotension from cardiac arrest.
A followup ECG was recorded 2 days later:
The patient's heart had significant recovery:
Echo:
NOTE: There are several subtle ECG findings in ECG #1.
ANSWER: Using the long lead II rhythm strip at the bottom in Figure-1 reveals that QRS morphology for the 7 beats in this tracing is not consistent — and this is the reason it's harder to define the J-point. I illustrate the additional subtle ECG findings I alluded to earlier for ECG #1 in Figure-3:
Our THANKS to Dr. Smith for presenting this fascinating case with fortunately, a positive ending.
She was unable to be defibrillated but was cannulated and placed on ECMO in our Emergency Department (ECLS - extracorporeal life support). ECMO Flow was achieved after approximately 1 hour of high quality CPR.
After good ECMO flow was established, she was successfully defibrillated.
Here is her monitor rhythm:
She then had a 12-lead:
 |
| What do you think? |
There is sinus bradycardia with one PVC. There is "Shark Fin morphology"
I saw this and thought for certain that this was going to be an LAD or left main occlusion as etiology of arrest, and etiology of profound ST Elevation in I, II, aVL, and V3-V6, and ST depression in III, V1 and V2.
The K was normal.
Here is a case of ECMO defibrillation with near shark fin that was due to proximal LAD occlusion.
Angiography showed normal coronaries.
The etiology of arrest was not determined.
Troponin I rose to 44.1 ng/mL (equivalent to an hs trop I of 44,000 ng/L). There were no further troponins, so we do not know the peak. This is a troponin I level that is almost exclusively seen in STEMI. In this case, profound shock for 1 hour would result in the same degree of infarction.
I suspect this is Type 2 MI due to prolonged severe hypotension from cardiac arrest.
A followup ECG was recorded 2 days later:
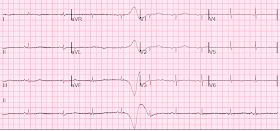 |
| No definite evidence of infarction. |
The patient's heart had significant recovery:
Echo:
Estimated LVEF 32%, apical wall motion abnormality with diastolic distortion (LV aneurysm), suggestive of old MI.
It was uncertain whether this represented:
1. A new acute MI (MINOCA -- see below -- which cause the arrest), or
2. An acute MI type 2 MI superimposed on an old MI, the scar from which would be the nidus of primary ventricular fibrillation. The Type 2 MI would then have been a result of the prolonged severe shock while in arrest.
So this is either a case of MINOCA, or a case of Type II STEMI.
1. If the arrest was caused by acute MI due to plaque rupture, then the diagnosis is MINOCA.
2. If the arrest had another etiology (such as old scar), and the ST elevation is due to severe shock, then it is a type II STEMI.
I believe the latter (type II STEMI) is most likely.
(MINOCA: Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease).
It was uncertain whether this represented:
1. A new acute MI (MINOCA -- see below -- which cause the arrest), or
2. An acute MI type 2 MI superimposed on an old MI, the scar from which would be the nidus of primary ventricular fibrillation. The Type 2 MI would then have been a result of the prolonged severe shock while in arrest.
So this is either a case of MINOCA, or a case of Type II STEMI.
1. If the arrest was caused by acute MI due to plaque rupture, then the diagnosis is MINOCA.
2. If the arrest had another etiology (such as old scar), and the ST elevation is due to severe shock, then it is a type II STEMI.
I believe the latter (type II STEMI) is most likely.
(MINOCA: Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease).
What is MINOCA?
Here is my comment on MINOCA:
"Non-obstructive coronary disease" does not necessarily imply "no plaque rupture with thrombus." Many non-obstructive plaques can fissure, thrombose, result in occlusion or near occlusion, have auto-lysis (spontaneous lysis of thrombus with reperfusion) and have less than 50% obstruction at angiography. They often cannot even be recognized as culprits, as fissured or ulcerated plaque.
If there is no coronary disease whatsoever on angiography (which is a lumenogram), then it is unlikely that there was a ruptured plaque. The ways to tell for certain include intravascular ultrasound (to look for extra-luminal plaque with rupture) or "optical coherence tomography," something I am entirely unfamiliar with.
This is a link to a scientific statement of MINOCA (full text pdf)
Here is my comment on MINOCA:
"Non-obstructive coronary disease" does not necessarily imply "no plaque rupture with thrombus." Many non-obstructive plaques can fissure, thrombose, result in occlusion or near occlusion, have auto-lysis (spontaneous lysis of thrombus with reperfusion) and have less than 50% obstruction at angiography. They often cannot even be recognized as culprits, as fissured or ulcerated plaque.
If there is no coronary disease whatsoever on angiography (which is a lumenogram), then it is unlikely that there was a ruptured plaque. The ways to tell for certain include intravascular ultrasound (to look for extra-luminal plaque with rupture) or "optical coherence tomography," something I am entirely unfamiliar with.
This is a link to a scientific statement of MINOCA (full text pdf)
Here are key points to take away from this summary, as published in this article at acc.org:
Diagnosis and Management of MINOCA Patients
The following are key points to remember from this American Heart Association Scientific Statement on the diagnosis and management of myocardial infarction in the absence of obstructive coronary artery disease (MINOCA):
- MINOCA occurs in 5-6% of acute myocardial infarction (AMI) cases (range reported between 5-15%).
- Patients with MINOCA are often younger, more likely to be women, and less likely to have dyslipidemia.
- Diagnosis of MINOCA should be made according to the Fourth Universal Definition of MI, in the absence of obstructive coronary artery disease (CAD) (no lesion ≥50%).
- The diagnosis of MINOCA should exclude: 1) other overt causes for elevated troponin (e.g., pulmonary embolism, sepsis, etc.), 2) overlooked obstructive coronary disease (e.g., distal stenosis or occluded small branches), and 3) nonischemic causes for myocyte injury (e.g., myocarditis).
- Nonobstructive coronary disease by coronary angiography should be differentiated between patients with normal coronary arteries and minimal luminal irregularities (less than 30% stenosis) and mild to moderate coronary atherosclerosis (30% to less than 50%). FFR can be useful. Takotsubo syndrome should be considered separately since it is not considered an MI by the Fourth Universal Definition of MI.
- Plaque disruption is common in MINOCA and encompasses plaque rupture, plaque erosion, and calcific nodules. The authors recommend using optical coherence tomography or intravascular ultrasound imaging in patients with evidence of nonobstructive CAD by angiogram.
- Coronary vasospasm is another common cause of MINOCA, defined as >90% vasoconstriction of an epicardial coronary artery resulting in compromised coronary blood flow.
- The gold standard technique for diagnosing coronary spasm is administration of high-dose intracoronary acetylcholine boluses with the response evaluated by invasive contrast angiography.
- Coronary microvascular dysfunction may contribute to MINOCA and requires further investigation.
- Coronary thrombosis or embolism can result in MINOCA, either with or without a hypercoagulable state.
- Spontaneous coronary artery dissection (SCAD) should be considered as a cause of MINOCA.
- Management of MINOCA is based on limited evidence and there are no prospective, randomized, controlled trials. Medications (aspirin, statin, beta-blockers, clopidogrel, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers) should be considered on the basis of the underlying mechanism for MINOCA in each individual. If there is any evidence of atherosclerosis, modifiable CAD risk factors should be treated aggressively.
- Coronary vasospasm is best treated with calcium channel blockers, and the benefits of long-acting nitrates are less clear.
- Additional research about MINOCA is needed to determine the efficacy of medications aimed at secondary prevention of AMI.
What is Type 2 MI?
It is ischemic injury caused by a mismatch of supply and demand, NOT by plaque rupture and thrombus. A list of conditions that can cause this is below.
Here is a great review article on Type 2 MI (full text, JACC) by my research partner, Yader Sanoval:
https://www.sciencedirect.com/science/article/pii/S0735109719305923
Here is a list from the 4th Universal Definition of MI (full text pdf):
https://www.onlinejacc.org/content/accj/72/18/2231.full.pdf?download=true
It is ischemic injury caused by a mismatch of supply and demand, NOT by plaque rupture and thrombus. A list of conditions that can cause this is below.
Here is a great review article on Type 2 MI (full text, JACC) by my research partner, Yader Sanoval:
https://www.sciencedirect.com/science/article/pii/S0735109719305923
Here is a list from the 4th Universal Definition of MI (full text pdf):
https://www.onlinejacc.org/content/accj/72/18/2231.full.pdf?download=true
===================================
MY Comment by KEN GRAUER, MD (5/19/2020):
===================================
Always nice to see successful stories like this one of recovery following resuscitation. As per Dr. Smith — “Shark Fin” morphology was noted on the initial monitoring strip, and initial 12-lead ECG.
- Shark Fin morphology has been discussed a number of times on Dr. Smith’s ECG Blog (For review — See the June 11, 2018 post and the January 24, 2020 post, to name just 2 instances).
For clarity — I’ll again show the initial ECG done in the ED in Figure-1.
- As per Dr. Smith — I also thought cardiac cath would show acute LAD or LMain OMI, instead of normal coronary arteries.
 |
| Figure-1: The initial 12-lead ECG in this case (See text). |
NOTE: There are several subtle ECG findings in ECG #1.
- Did YOU see them? IF not — Please take another look at Figure-1.
COMMENT: I’ll emphasize that the following subtleties in ECG interpretation of the tracing in Figure-1 do not change management — and they all resolved by the time the follow-up tracing 2 days later was done. But I found these points interesting — and I hope they help to hone your ECG interpretation skills.
- The 1st challenge in interpreting a tracing with Shark Fin morphology — is to define the J-point that separates the end of the QRS complex with the beginning of the ST segment. As Drs. Meyers & Smith have often said — “When the QRS is wide, the J-point will hide. So, your next step is to Trace it down, and Copy it over."
- To illustrate this process — I’ve reproduced in Figure-2 the initial ECG from the Jan. 24, 2020 post (link to that post given above). Note that the rhythm in Figure-2 is sinus with a PVC ( = beat #10) — and, that there is bifascicular block (RBBB/LAHB), as is commonly seen with the proximal LAD occlusion that was found at cath. The location of the J-point is easiest to see in the limb leads. I’ve traced this down in Figure-2 with vertical BLUE lines that reveal the dramatic ST elevation in the anterior leads.
 |
| Figure-2: The initial ECG from the January 24, 2020 post — to illustrate how to define the J-point when there is Shark Fin morphology from marked ST segment elevation (See text). |
Go back to Figure-1.
- WHY is it more difficult to define the J-point in the ECG for this case?
ANSWER: Using the long lead II rhythm strip at the bottom in Figure-1 reveals that QRS morphology for the 7 beats in this tracing is not consistent — and this is the reason it's harder to define the J-point. I illustrate the additional subtle ECG findings I alluded to earlier for ECG #1 in Figure-3:
- P waves are present in the long lead II rhythm strip in Figure-3 (RED arrows). Note that the PR interval does not remain the same for each of the 7 beats in this lead II rhythm strip. However, the PR interval does remain the same (albeit prolonged to ~0.32 second) for beats #1, 5 and 7 — which tells us that beats #1, 5 and 7 are being conducted with 1st-degree AV block.
- The QRS complex of these 3 beats ( = beats #1, 5, 7) that are conducted with 1st-degree AV block looks narrower, with a more defined end point of the QRS — compared to the more amorphous QRST complexes of beats #2, 3 and 6. No P wave precedes beat #4.
- Note that the PR interval preceding beats #2, 3 and 6 is clearly shorter than the PR interval of the 3 beats conducted with 1st-degree AV block. Looking further — the PR interval preceding beat #3 is shorter than the PR interval preceding beats #2 and #6.
- PEARL: In my experience, cardiac rhythms observed during (or shortly after) cardiac arrest do not obey the usual rules for AV blocks and escape rhythms. In non-arrest situations — escape beats and escape rhythms tend to be at least fairly regular. This is an extremely helpful concept to appreciate — because recognizing that a beat occurs earlier-than-expected (ie, earlier than other escape beats in the tracing) — is usually an excellent clue that this beat is conducted. That said, the R-R interval for the 7 beats in the lead II rhythm strip in Figure-3 varies in a way that to me defies logic.
- BOTTOM LINE: Beats #1, 5 and 7 in Figure-3 are conducted with 1st-degree AV block. The vertical BLUE lines in this figure define the J-point, and reveal dramatic ST elevation in antero-lateral leads. I don’t know what beat #4 is (ie, No P wave precedes this beat). I’m not sure if beats #2, 3 and 6 are fusion beats — or if these represent ventricular escape beats preceded by non-conducting P waves (or a combination of these possibilities). Regardless, dramatic ST elevation is seen in the antero-lateral leads of all beats (conducted and/or escape) on this tracing. And, results of cardiac cath revealed normal coronary arteries — with a follow-up ECG recorded 2 days later showing a sinus mechanism with complete resolution of all ST elevation.
 |
| Figure-3: I've labeled the initial ECG in this case to highlight several additional subtle findings in ECG #1 (See text). |
Our THANKS to Dr. Smith for presenting this fascinating case with fortunately, a positive ending.



Thanks doc for your teaching
ReplyDeleteYou are welcome!
DeleteThanks a lot Sir, for sharing these insightful cases!
ReplyDeleteOur pleasure! — :)
DeleteFantastic case sir. Take a bow to u all
ReplyDeleteTHANK YOU — :)
Delete