A 40-something male presents to the stabilization room for evaluation following head on motor vehicle collision (MVC). Pt was reported restrained driver, hit at city speeds, with + airbag deployment.
The MVC was unquestionably caused by the other car, not by this driver.
The patient complained to EMS of chest pain and a prehospital EKG en route was concerning for STEMI.
The patient was at all times hemodynamically stable, without evidence of any profuse bleeding.
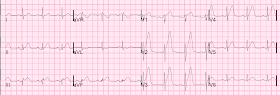
He had an ECG recorded on arrival to the ED:
Further patient history revealed that the chest pain started following the crash, and that he had no chest pain previously. Brief review of chart showed no h/o known CAD. DDx was possible aortic dissection or injury, cardiac contusion, LAD dissection, or type I MI w/ plaque rupture.
They performed a bedside ED cardiac ultrasound:
With this ECG, there must be a wall motion abnormality if the ECG and echo are done at the same time.
If you do not see a wall motion abnormality, it is because:
1. ultrasound, or your technique or expertise, is not good enough.
2. The artery reperfused quickly enough, such that all wall motion recovered.
It is possible to have a normal echo if, immediately after the ECG:
1. The artery reperfuses and
2. The duration of ischemia was short.
However, this was the provider's interpretation:
"Further bedside ultrasound of cardiac function w/o demonstration of significant WMA or impaired function."
The patient went to pan scan, with CT of chest/abd/pelvis. It showed a splenic laceration and pelvic fracture, and also this:
When the patient returned to the ED, he had another ED cardiac ultrasound:
This echo was done with Speckle Tracking, Strain Echocardiography. This was done by one of the most expert ED echocardiographers in the world:
At this point, another ECG should have been recorded, but was not. It would almost certainly have shown resolution of the STEMI findings (due to spontaneous reperfusion).
Should the cath lab have been activated?
This is a tough question. PCI usually requires antiplatelet and antithrombotic therapy, but a persistently occluded LAD must be opened, even if such medications cannot be given. Plain old balloon angioplasty without these meds can temporarily open the artery.
I asked our chief of cardiology, Gautam Shroff: "Plain old balloon angioplasty (POBA) requires at least one antiplatelet agent indefinitely and 2 of them for 30 days, or else it clots off. Heparin is also needed during the cath. For a head bleed I would sacrifice the anterior wall. Perhaps the best approach for this case would be splenectomy (or Interventional radiology to stop the bleed?), then POBA or bare metal stent?? No good options and risk benefit has to be weighed."
So Cardiology consultation is essential, and coordination with trauma surgery and interventional radiology.
Fortunately for this patient, as you'll see below, and unbeknownst to the providers, the artery quickly spontaneously reperfused.
FINAL ED DIAGNOSES:
1. MVC
2. Splenic laceration
3. Pelvic fracture
4. ST elevation w/ chest pain
The patient was admitted to the hospital. He did have serial troponins:
At this point (8 hours later), the 2nd ECG was recorded:
Formal echo was done:
Normal left ventricular cavity size.
Normal estimated left ventricular ejection fraction - 70%.
Regional wall motion abnormality- apex, distal septum and inferior segments.
This demonstrates that a bubble contrast echo, read by an expert, is superior at identifying subtle wall motion abnormalities than an ED echo.
Another 24 hours of troponins were recorded, starting at time 1504:
This was recorded another 24 hours later:
Angiogram, 5 days later:
--Culprit for the patient's "NSTEMI" (!) [comment: It is really a transient STEMI) is plaque rupture or traumatic coronary dissection in the distal LAD. It is a wraparound LAD to the inferior wall.
--While there is residual 80-90% stenosis, there is TIMI III flow beyond
--Will initiate treatment with clopidogrel (300 mg give post-procedure), without PCI at this time, given potential inability to tolerate DAPT in the setting of splenic laceration/rupture.
--Can proceed to PCI if patient tolerates anticoagulation and develops angina prior to discharge or would plan for staged re-look angiography and PCI in 4-6 weeks.
Summary:
So this was a brief LAD occlusion, with transient STEMI. It may have been a dissection related to the trauma, or coincidental plaque rupture, which could be triggered by the stress of the trauma.
Here are several cases of Takotsubo with ST Elevation that could mimic STEMI.
Many takotsubo have on T-wave inversion, in which case emergent cath lab activation is not critical (though it could mimic Wellens')
The MVC was unquestionably caused by the other car, not by this driver.
The patient complained to EMS of chest pain and a prehospital EKG en route was concerning for STEMI.
The patient was at all times hemodynamically stable, without evidence of any profuse bleeding.
He had an ECG recorded on arrival to the ED:
Further patient history revealed that the chest pain started following the crash, and that he had no chest pain previously. Brief review of chart showed no h/o known CAD. DDx was possible aortic dissection or injury, cardiac contusion, LAD dissection, or type I MI w/ plaque rupture.
They performed a bedside ED cardiac ultrasound:
With this ECG, there must be a wall motion abnormality if the ECG and echo are done at the same time.
If you do not see a wall motion abnormality, it is because:
1. ultrasound, or your technique or expertise, is not good enough.
2. The artery reperfused quickly enough, such that all wall motion recovered.
It is possible to have a normal echo if, immediately after the ECG:
1. The artery reperfuses and
2. The duration of ischemia was short.
This shows an apical wall motion abnormality
This is consistent with anterior/inferior MI due to wraparound LAD, and also with takotsubo.
This is consistent with anterior/inferior MI due to wraparound LAD, and also with takotsubo.
However, this was the provider's interpretation:
"Further bedside ultrasound of cardiac function w/o demonstration of significant WMA or impaired function."
The patient went to pan scan, with CT of chest/abd/pelvis. It showed a splenic laceration and pelvic fracture, and also this:
Just the heart is shown from the CT of chest/abdomen/pelvis, with contrast
 |
| Shows a clear apical area without any perfusion (dark, no contrast gets to this myocardium) |
Slightly different CT technique:
 |
| Again, apical area without contrast |
These images further confirm the diagnosis of acute STEMI.
When the patient returned to the ED, he had another ED cardiac ultrasound:
Now there is no clear wall motion abnormality, but these can be very difficult to appreciate.
This echo was done with Speckle Tracking, Strain Echocardiography. This was done by one of the most expert ED echocardiographers in the world:
Good contraction is measured by a large negative value. The light green is the most negative, and this would represent the most wall contraction, and represents the lateral wall. Red, which is the apex, has moderate contraction, so there may be a subtle apical wall motion abnormality still.
But it is very hard to appreciate with the naked eye, without speckle tracking.
At this point, another ECG should have been recorded, but was not. It would almost certainly have shown resolution of the STEMI findings (due to spontaneous reperfusion).
Should the cath lab have been activated?
This is a tough question. PCI usually requires antiplatelet and antithrombotic therapy, but a persistently occluded LAD must be opened, even if such medications cannot be given. Plain old balloon angioplasty without these meds can temporarily open the artery.
I asked our chief of cardiology, Gautam Shroff: "Plain old balloon angioplasty (POBA) requires at least one antiplatelet agent indefinitely and 2 of them for 30 days, or else it clots off. Heparin is also needed during the cath. For a head bleed I would sacrifice the anterior wall. Perhaps the best approach for this case would be splenectomy (or Interventional radiology to stop the bleed?), then POBA or bare metal stent?? No good options and risk benefit has to be weighed."
So Cardiology consultation is essential, and coordination with trauma surgery and interventional radiology.
Fortunately for this patient, as you'll see below, and unbeknownst to the providers, the artery quickly spontaneously reperfused.
FINAL ED DIAGNOSES:
1. MVC
2. Splenic laceration
3. Pelvic fracture
4. ST elevation w/ chest pain
The patient was admitted to the hospital. He did have serial troponins:
First 3 troponins
 |
| Notice the last one shoots up, which is typical with reperfusion (artery opening, resulting in sudden troponin release). |
At this point (8 hours later), the 2nd ECG was recorded:
 |
| ST Elevation is gone. There are Q-waves in V2-V4. The LAD has reperfused. |
Formal echo was done:
Normal left ventricular cavity size.
Normal estimated left ventricular ejection fraction - 70%.
Regional wall motion abnormality- apex, distal septum and inferior segments.
This demonstrates that a bubble contrast echo, read by an expert, is superior at identifying subtle wall motion abnormalities than an ED echo.
Another 24 hours of troponins were recorded, starting at time 1504:
This was recorded another 24 hours later:
 |
| Terminal and symmetric deep T-wave inversion (reperfusion T-waves), with Q-waves (not exactly Wellens' waves, which should have R-wave preservation) |
All troponins
 |
| It is really not necessary to get all these troponins, but they are interesting. |
Angiogram, 5 days later:
--Culprit for the patient's "NSTEMI" (!) [comment: It is really a transient STEMI) is plaque rupture or traumatic coronary dissection in the distal LAD. It is a wraparound LAD to the inferior wall.
--While there is residual 80-90% stenosis, there is TIMI III flow beyond
--Will initiate treatment with clopidogrel (300 mg give post-procedure), without PCI at this time, given potential inability to tolerate DAPT in the setting of splenic laceration/rupture.
--Can proceed to PCI if patient tolerates anticoagulation and develops angina prior to discharge or would plan for staged re-look angiography and PCI in 4-6 weeks.
Summary:
So this was a brief LAD occlusion, with transient STEMI. It may have been a dissection related to the trauma, or coincidental plaque rupture, which could be triggered by the stress of the trauma.
Here are several cases of Takotsubo with ST Elevation that could mimic STEMI.
Many takotsubo have on T-wave inversion, in which case emergent cath lab activation is not critical (though it could mimic Wellens')
Diffuse ST Elevation with Apical Ballooning: is it Takotsubo Stress Cardiomyopathy?
Takotsubo Stress Cardiomyopathy, with Echocardiogram
Here are some cases of myocardial contusion