This is a case written by Dan Lee (One of our fantastic Hennepin Residents, class of 2020)
edits by Smith
A 60 something-year-old man with a history of ESRD, LVH and prior CABG presented after an episode of hypotension during his hemodialysis, run followed by a syncopal episode which caused his run to be terminated early. His other symptoms on presentation were general lethargy and mild shortness of breath. No chest pain. His vitals were initially normal.
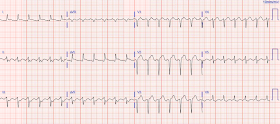
We will start with his baseline EKG:
This is his presenting EKG (T= 26min):
What do you think?
Presenting EKG (T= 26min):
Compared to the baseline EKG which looks fairly typical of normal LVH, this presenting EKG has what we call “pseudonormalization” of the ST segments in V2 and V3 - the ST elevation that was once present at baseline is now "normalizing", to the point where there is virtually no elevation in V3. Hence, the term "pseudonormalization", because it is not really normal - LVH should have repolarization changes seen in the baseline EKG. Thus, there is relative ST depression. It is analogous to a normal EKG having ST depression in V2 and V3, which would be abnormal and concerning for posterior MI in the right clinical context. But because of LVH, this is masked in this patient. There is also ST depression in the inferior leads - this was present on the old EKG, but there is also NEW ST depression in aVL.
Closer look at V2 and V3 side by side:
Furthermore, in both V2 and V3, the ST segment has flattened, resulting in more concavity of the ST-T complex. Normally, concavity in ST segments suggests absence of anterior ischemia (though concavity by itself is not reassuring - see this study). But in this case, the concavity is actually more concerning because now we are worried about posterior MI. Given the inverted voltage from the posterior myocardium that we see on a normal EKG, deepening concavity would be analogous to elevating convexity in the posterior myocardium.
Same image above with V3 inverted and flipped on its axis to better visualize the posterior ST segment:
The patient's initial troponin I was 2.0 ng/mL (99% reference level = 0.030 ng/mL. With his ESRD, he does have an elevated baseline troponin at ~0.40 ng/mL. His ED cardiac ultrasound (which is not at all ideal for detecting wall motion abnormalities, and is also very operator dependent for this finding) was significant for depressed global EF. His prior EF from an ECHO 6 months prior indicated 35% LVEF.
What would you do in this scenario?
I think a good start would be a posterior EKG and a high quality contrast echocardiogram read by an expert. Unfortunately, neither were done in this case.
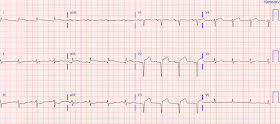
This was the patient's second EKG in the ED, 3 hours after the first (T= 217min):
The patient's symptoms were attributed to probable hypovolemia secondary to poor oral intake and to a UTI, but given his troponin elevation he was admitted on a heparin drip and serial troponins due to his high risk history.
edits by Smith
A 60 something-year-old man with a history of ESRD, LVH and prior CABG presented after an episode of hypotension during his hemodialysis, run followed by a syncopal episode which caused his run to be terminated early. His other symptoms on presentation were general lethargy and mild shortness of breath. No chest pain. His vitals were initially normal.
 |
| There is voltage suggestive of LVH. There are appropriate repolarization changes secondary to the LVH, including 1-2mm of ST elevation in V2 and V3. |
This is his presenting EKG (T= 26min):
Presenting EKG (T= 26min):
Compared to the baseline EKG which looks fairly typical of normal LVH, this presenting EKG has what we call “pseudonormalization” of the ST segments in V2 and V3 - the ST elevation that was once present at baseline is now "normalizing", to the point where there is virtually no elevation in V3. Hence, the term "pseudonormalization", because it is not really normal - LVH should have repolarization changes seen in the baseline EKG. Thus, there is relative ST depression. It is analogous to a normal EKG having ST depression in V2 and V3, which would be abnormal and concerning for posterior MI in the right clinical context. But because of LVH, this is masked in this patient. There is also ST depression in the inferior leads - this was present on the old EKG, but there is also NEW ST depression in aVL.
Closer look at V2 and V3 side by side:
Furthermore, in both V2 and V3, the ST segment has flattened, resulting in more concavity of the ST-T complex. Normally, concavity in ST segments suggests absence of anterior ischemia (though concavity by itself is not reassuring - see this study). But in this case, the concavity is actually more concerning because now we are worried about posterior MI. Given the inverted voltage from the posterior myocardium that we see on a normal EKG, deepening concavity would be analogous to elevating convexity in the posterior myocardium.
Same image above with V3 inverted and flipped on its axis to better visualize the posterior ST segment:
 |
| This is inverted: it is as if you were looking from the posterior wall. The baseline is on the left, and now it looks as if the ST segment is rising, with less downsloping. |
The patient's initial troponin I was 2.0 ng/mL (99% reference level = 0.030 ng/mL. With his ESRD, he does have an elevated baseline troponin at ~0.40 ng/mL. His ED cardiac ultrasound (which is not at all ideal for detecting wall motion abnormalities, and is also very operator dependent for this finding) was significant for depressed global EF. His prior EF from an ECHO 6 months prior indicated 35% LVEF.
What would you do in this scenario?
I think a good start would be a posterior EKG and a high quality contrast echocardiogram read by an expert. Unfortunately, neither were done in this case.
This was the patient's second EKG in the ED, 3 hours after the first (T= 217min):
 |
| Deeper and more downsloping ST in V5 and V6 There is also pseudonormalization of the previously inverted T-wave in aVL |
The patient's symptoms were attributed to probable hypovolemia secondary to poor oral intake and to a UTI, but given his troponin elevation he was admitted on a heparin drip and serial troponins due to his high risk history.
His inpatient clinicians did not think that an urgent angiogram was warranted given that he was chest pain free, his EKG appeared nondiagnostic, and serial troponins were not elevating beyond 2 ug/L.
Later on during the night of his admission he had a short episode of chest pain that resolved with sublingual nitroglycerin.
Around midnight, the patient became more hypotensive with systolic BP in the 60s, and was complaining of feeling nauseous and "sick to his stomach".
He had another EKG recorded at this time (T = 487 min):
Around 4AM, the patient had worsening dyspnea and hypoxia with continued hypotension.
He became confused and had another EKG done (T = 899 min):
Shortly after this EKG, his HR dropped into the 40's and he subsequently had a PEA arrest requiring 1 round of chest compressions, followed by ROSC. He was taken to the cath lab at that time (T = 1024 min).
He was found to have a 100% occlusion of the proximal anastomotic portion of a prior SVG from the aorta to the OM1 which in turn had a vein graft to the distal RCA. There was initially TIMI 0 flow . The lesion was intervened on with balloon angioplasty and had subsequent TIMI 3 flow. It was thought to be an in stent restenosis and thrombosis from a DES placed in the same region 6 months prior.
His troponin I peaked at 97 ng/mL (very large MI!).
His follow up ECHO the next day revealed an EF of 24% and a posterior wall motion abnormality. Fortunately, he was extubated several days later in the ICU with intact baseline mental status and was discharged shortly thereafter to subacute rehab.
---------
Acute myocardial infarction in patients with dialysis
Patients on dialysis have a well studied history of underdiagnosis and undertreatment for acute myocardial infarction. This is paradoxical given the absurdly high 2 year mortality rate of 70% after a MI in dialysis patients.
This study from Herzog et al (from our own Hennepin County Medical Center) included patients from a national registry and compared 3049 patients on dialysis admitted and eventually found to have acute MI compared with 534,395 patients not on dialysis admitted with an eventual diagnosis of acute MI. Of these groups, only 22% of dialysis patients had an admission diagnosis consistent with acute MI while 43.8% of nondialysis patients had the correct admission diagnosis of acute MI. Dialysis patients had double the rate of cardiac arrest (11% vs 5%), were less likely to receive reperfusion therapy when eligible (47% vs. 75%), and had an increased odds ratio of death compared to nondialysis patients 1.5 (95% CI 1.3-1.7).
Why is this? One reason for underdiagnosis might be that only 44% of these dialysis patients presented with chest pain while over 68% of those not on dialysis had chest pain on presentation. Another reason may be that the EKG is more difficult to interpret in patients with dialysis due to baseline abnormalities, including LVH. In this study of dialysis patients with severe CAD, 77% had an abnormal resting EKG and the most common abnormality was LVH.
Herzog et al. comment on this latter study: “There is a lower index of clinical suspicion and a higher level of inaccuracy for initial diagnosis of acute coronary syndromes in dialysis patients with AMI because twice as many patients were incorrectly diagnosed on admission. Further diagnostic confusion may have resulted from the greater prevalence of hypertension and likely attendant hypertensive heart disease and uninterpretable ST depression in the dialysis cohort.”
What can we learn?
1. Occlusion Myocardial Infarction (OMI) often does not present with diagnostic ST elevation, or even any STE, especially in dialysis patients.
2. Pseudonormalization of ST segments, and also of T-waves, may be a useful sign to help detect OMI in the setting of LVH.
3. Patients on dialysis often do not have chest pain in the setting of acute MI. Have a high index of suspicion for MI in these patients and advocate for them.
3. Think about, and use, emergent contrast echocardiography more liberally.
Later on during the night of his admission he had a short episode of chest pain that resolved with sublingual nitroglycerin.
Around midnight, the patient became more hypotensive with systolic BP in the 60s, and was complaining of feeling nauseous and "sick to his stomach".
He had another EKG recorded at this time (T = 487 min):
 |
| More subtle flattening of ST segment in V2 and V3. |
Around 4AM, the patient had worsening dyspnea and hypoxia with continued hypotension.
He became confused and had another EKG done (T = 899 min):
Shortly after this EKG, his HR dropped into the 40's and he subsequently had a PEA arrest requiring 1 round of chest compressions, followed by ROSC. He was taken to the cath lab at that time (T = 1024 min).
He was found to have a 100% occlusion of the proximal anastomotic portion of a prior SVG from the aorta to the OM1 which in turn had a vein graft to the distal RCA. There was initially TIMI 0 flow . The lesion was intervened on with balloon angioplasty and had subsequent TIMI 3 flow. It was thought to be an in stent restenosis and thrombosis from a DES placed in the same region 6 months prior.
His troponin I peaked at 97 ng/mL (very large MI!).
His follow up ECHO the next day revealed an EF of 24% and a posterior wall motion abnormality. Fortunately, he was extubated several days later in the ICU with intact baseline mental status and was discharged shortly thereafter to subacute rehab.
---------
Acute myocardial infarction in patients with dialysis
Patients on dialysis have a well studied history of underdiagnosis and undertreatment for acute myocardial infarction. This is paradoxical given the absurdly high 2 year mortality rate of 70% after a MI in dialysis patients.
This study from Herzog et al (from our own Hennepin County Medical Center) included patients from a national registry and compared 3049 patients on dialysis admitted and eventually found to have acute MI compared with 534,395 patients not on dialysis admitted with an eventual diagnosis of acute MI. Of these groups, only 22% of dialysis patients had an admission diagnosis consistent with acute MI while 43.8% of nondialysis patients had the correct admission diagnosis of acute MI. Dialysis patients had double the rate of cardiac arrest (11% vs 5%), were less likely to receive reperfusion therapy when eligible (47% vs. 75%), and had an increased odds ratio of death compared to nondialysis patients 1.5 (95% CI 1.3-1.7).
Why is this? One reason for underdiagnosis might be that only 44% of these dialysis patients presented with chest pain while over 68% of those not on dialysis had chest pain on presentation. Another reason may be that the EKG is more difficult to interpret in patients with dialysis due to baseline abnormalities, including LVH. In this study of dialysis patients with severe CAD, 77% had an abnormal resting EKG and the most common abnormality was LVH.
Herzog et al. comment on this latter study: “There is a lower index of clinical suspicion and a higher level of inaccuracy for initial diagnosis of acute coronary syndromes in dialysis patients with AMI because twice as many patients were incorrectly diagnosed on admission. Further diagnostic confusion may have resulted from the greater prevalence of hypertension and likely attendant hypertensive heart disease and uninterpretable ST depression in the dialysis cohort.”
What can we learn?
1. Occlusion Myocardial Infarction (OMI) often does not present with diagnostic ST elevation, or even any STE, especially in dialysis patients.
2. Pseudonormalization of ST segments, and also of T-waves, may be a useful sign to help detect OMI in the setting of LVH.
3. Patients on dialysis often do not have chest pain in the setting of acute MI. Have a high index of suspicion for MI in these patients and advocate for them.
3. Think about, and use, emergent contrast echocardiography more liberally.
-----------------------------------------------------------
Comment by KEN GRAUER, MD (10/1/2018):
-----------------------------------------------------------
Excellent case presentation by Dr. Dan Lee — with superb insight into the subtleties (and true “art”) of clinical electrocardiography. THANK YOU Dr. Lee! In the interest of enhancing several important concepts in clinical ECG interpretation — I focus my comments on a number of points not elicited in the above presentation.
- Essential to the clinical deductions made in this case was access to a prior baseline tracing on this patient. This critical point is often overlooked. Even when available — without meticulous lead-by-lead comparison — subtle ECG changes are easy to miss ...
- For clarity, I have put the first 3 ECGs shown in this case together in Figure-1:
My approach to comparing a current tracing with a prior one — is to begin by completely interpreting one of the 2 tracings. ONLY THEN — do I begin lead-to-lead comparison to assess which differences might be clinically relevant.
- For optimal comparison — it is essential to ensure that you are “comparing apples with apples — and not with oranges”. By this I mean, that not only ST-T wave appearance needs to be assessed — but also QRS appearance (relative size of R and S waves, as well as total voltage) — R wave progression (Does transition occur at the same point in the chest leads of both tracings?) — and frontal plane axis (Is there a difference in axis between the 2 tracings?).
- NOTE: Much of the time, there will be at least some differences in QRS appearance, transition and/or frontal plane axis. This does not mean that lead-to-lead comparison cannot be done — but rather: i) that the interpreter BE aware that precise comparison of ST-T wave appearance is not the entire story — and, that it may not be completely reflective of whether or not true ST-T wave changes have occurred vs a difference in ST-T wave appearance as the result of a shift in axis or electrode lead placement, or the patient now being supine compared to a previous inclined position; and, ii) that the interpreter needs to “factor in” whatever axis or QRS changes have occurred in their assessment as to whether ST-T wave changes are (or are not) likely to be significant. Herein lies the “ART” of electrocardiography.
=========================
Take another look at this patient’s baseline tracing (= TOP ECG in Figure-1). How would YOU interpret this tracing?
- Is there evidence of possible prior infarction?
- How likely is it that this patient has LVH? (ie, Is it more than 50-60% likely?).
- In there ECG evidence of possible ongoing ischemia? (ie, What IF this “baseline tracing” was the only ECG you had — and it was obtained from a patient describing new chest discomfort? In this case — Could any of the changes be acute?).
 |
| Figure-1: The first 3 ECGs shown in this case (See text). |
ANSWER: The rhythm in the baseline tracing is sinus at ~75/minute. The PR, and QRS intervals look normal normal. The QTc looks to be slightly prolonged. The axis is normal (about +50 degrees). Q waves are present in each of the 3 inferior leads. This may reflect prior inferior infarction. A small (probably septal) q wave is present in V6. Regarding chamber enlargement — the unusually pointed P wave in lead V2 is consistent with RAA (Right Atrial Abnormality). Artifact prevents assessment of the P wave in lead V1 for LAA (Left Atrial Abnormality). There is dramatic increase in QRS voltage in multiple chest leads (with overlap of QRS and S waves in leads V3,V5,V6).
- ST-T wave changes in lateral chest leads are clearly consistent with LV “strain” — which together with the clinical history + dramatic increase in QRS amplitude makes the likelihood of true chamber LVH >98%.
- In addition — there is J-point depression in leads V5,V6 with ST segment coving in these leads. T wave inversion in lead V5 is clearly more symmetric than is usually seen with just LV “strain”. ST segment coving with slight, symmetric T wave inversion is also seen in leads I and aVL — and inferior leads clearly show ST-T wave flattening.
- IMPRESSION: I’d interpret this tracing as consistent with LVH and/or “strain” and/or ischemia. I’d suspect that these changes are probably not acute, but rather consistent with longstanding hypertension + renal failure — BUT — it’s important to be aware that: i) Clinical Correlation is needed in order to assess the ECG findings we see on this baseline tracing; and, ii) More than just LVH appears on this baseline tracing.
- NOTE: For those wanting review of ECG assessment for LAA, RAA and LVH (with “strain” and/or ischemia) — CLICK HERE (for LVH) — AND HERE (for RAA/LAA).
=========================
Drs. Lee and Smith have highlighted the important subtle differences in ST-T waves during sequential tracings in this case (especially evident with the superb blow-ups of leads V2,V3 in their presentation above).
Drs. Lee and Smith have highlighted the important subtle differences in ST-T waves during sequential tracings in this case (especially evident with the superb blow-ups of leads V2,V3 in their presentation above).
QUESTION: While I completely agree with the assessment by Drs. Lee and Smith, that there appear to be dynamic ST-T wave changes that are ongoing in this case in association with clinical symptoms — Take ANOTHER LOOK at the Baseline ECG and ECG #1 in the ED, as shown in Figure-1:
- Are we truly comparing “apples with apples” when we look at these 2 tracings?
- Now take another look at ECG #2 (Bottom tracing in Figure-1) — and compare it with ECG #1 in this Figure. Your Comment?
ANSWER: Clearly, there are differences in axis, QRS amplitude, and R wave progression between the Baseline ECG and ECG #1:
- Note the predominant negativity of the QRS complex in lead III of ECG #1 and the much smaller QRS in aVF ( = some axis shift has clearly occurred since the baseline tracing). In addition, lateral chest lead R waves are clearly less tall, and transition occurs later in ECG #1.
- NOTE: I completely agree with Drs. Lee and Smith that despite these differences in axis, amplitude and transition — the subtle changes of “pseudonormalization” are most probably real. My point is simply to highlight the importance of taking these differences in axis, amplitude and transition into account — and to realize that in some cases, such differences will make it much more difficult to assess the clinical significance of ST-T wave changes.
- Finally — Note that axis, QRS amplitude and transition in ECG #2 all look more like they did in the Baseline ECG. Yet despite this — inferior T waves are now all clearly inverted in ECG #2 (compared to the baseline ECG) — the ST coving and shallow T inversion in leads I and aVL have been replaced by flatter ST segments and upright T waves — and, the subtle ST-T wave “pseudonormalization” changes are still present in many of the chest leads. This supports the notion that there have been ongoing dynamic ST-T wave changes in this patient with clear indication for cardiac catheterization.