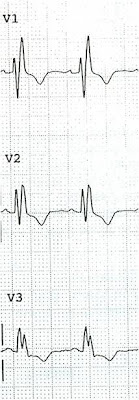
I recently received this from a reader:
"I recently saw this 31 year old male with central chest tightness that started at 530am and lasted 30 min. He came to ED pain free at 7 AM with no other symptoms. The initial ECG showed J point elevation in inferior leads. The morning doctors decided to do serial cardiac enzymes. The first trop I was negative and subsequently went up slightly. Patient was managed as NSTEMI. A few days later the coronary angiogram was completely normal. Please advise me on this ECG. Should we have done a bed side echo to look for wall motion abnormality and would that have made a difference in management?"
Here was my response:
"This is diagnostic of inferior injury (STEMI). There is inferior STE with hyperacute T waves, reciprocal ST depression and T-wave inversion in aVL. Also STE in V5 and V6. It spontaneously reperfused. A negative cath does not prove anything. There was either thrombus that completely lysed or coronary spasm. Did they do IVUS (intravascular ultrasound of coronaries)? That would identify extra coronary plaque as the source of ACS."
A high quality echocardiogram would most likely have shown a wall motion abnormality.
"If you can find a repeat ECG, it will show resolution of the findings on subsequent ecg's. Can you find one?"
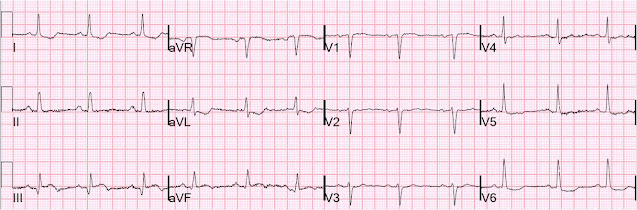
He did find one and here it is:
 |
Complete resolution of ST elevation
|
|
The troponin I peaked at 5. The angiogram was completely normal. The patient was treated with clopidogrel.
This was a reperfused STEMI.
Normal Coronaries in Suspected Acute Coronary Syndrome:
The 4 articles below, about 8% of cases referred for primary angioplasty of STEMI have completely normal coronaries. Many of these are false positive ECGs, but some are MI with due to spontaneous reperfusion. If there is spontaneous reperfusion, the ECG will always show typical evolution or resolution.
This case has an unequivocal ECG; it is clearly STEMI. In cases in which the ECG is not unequivocal, absence of change over time proves it is not a STEMI.
By the way:
1) Non STEMI but with ST elevation due to non-AMI etiologies (early repol, LVH, etc.) is never documented in these studies.
2) Furthermore, the mere presence of non-obstructive coronary disease, unless one sees a culprit lesion, does not prove that the symptoms were due to AMI.
Whether there is ACS or not, troponin will often be positive because troponin is nonspecific: it is elevated is many states including pulmonary embolism, myocarditis, stress cardiomyopathy, dilated cardiomyopathy, hypertensive cardiomyopathy, and more. This has recently been called a "type II MI".
Coronaries can now be evaluated with other means, such as intravascular ultrasound (IVUS). Even when there is stenosis, most atherosclerosis is extraluminal. It can be seen with IVUS, but not with a "lumenogram," which is what an angiogram is. These atherosclerotic plaques can cause intralumenal thrombosis with STEMI. If the clot lyses completely, the ischemia resolves and the angiogram is normal.
Bibliography, with edited abstracts
There were 821 cath lab activations and 86% were treated by mechanical revascularization. In 76 patients (8.5%), no coronary artery stenosis was documented. Observations documented angiographically included coronary spasms (6.6%) and muscle bridges (5.3%). During a mean follow-up of 11.2±6.4 months, one patient developed an acute myocardial infarction requiring coronary intervention. All other patients were free of any cardiac event.
Of 898 patients who had cath lab activations for primary PCI, normal coronary angiograms were obtained for 26 patients (2.6%). Among these, the diagnosis at discharge was a small myocardial infarction in seven patients (0.7%), acute (peri)myocarditis in five patients, dilated cardiomyopathy in four patients, hypertension with left ventricular hypertrophy in three patients, pulmonary embolism in two patients and misinterpretation of the electrocardiogram (ie, no cardiac disease) in five patients.
Seven patients with small infarctions underwent angiography within 30 min to 90 min of complete relief of the signs of acute ischemia, and thus, angiograms during pain were not taken. None of the 898 patients catheterized during ongoing symptoms of ischemia had a normal coronary angiogram. Spontaneous coronary spasm as the only cause (without underlying coronary atherosclerosis) for the evolving infarction was not seen. Thus, the causes of the seven small infarcts in patients with normal angiograms remain uncertain.
Characteristics of 690 consecutive patients with presumed STEMI referred for primary PCI. 87 (13%) had angiographically normal coronary arteries and were compared with patients with angiographically shown culprit lesions (control group; n = 594). Nine patients with significant coronary disease, but no identifiable culprit lesion, were excluded. Electrocardiograms (ECGs) from both groups were reviewed by 2 cardiologists blinded to angiographic findings. On expert review of ECGs, 55% of patients in the normal coronaries group had ST-elevation criteria for STEMI (vs 93% in the control group, but the ECG was considered consistent with a diagnosis of STEMI by both observers in only 33% (vs 92% in the control group) Left branch bundle block independently correlated with normal coronary arteries on multivariate analysis (odds ratio for STEMI 0.016). The discharge diagnosis in the normal coronaries group was predominantly pericarditis (n = 72; 83%), but these were not adjudicated by the authors. Other diagnoses were myocarditis in 3 patients (3%), Takotsubo cardiomyopathy in 2 patients (2%), presumed coronary spasm secondary to intravenous drug abuse in 2 patients (2%), cryptogenic AMI in 1 patient (1%), dilated cardiomyopathy in 1 patient (1%), massive pulmonary embolus in 1 patient (1%), cholelithiasis in 1 patient (1%), and pneumonia in 1 patient (1%).
The most likely alternative diagnosis suggested by both observers for the non-AMI ECGs in the normal coronaries group was normal variant ST changes (25% observer 1 and 26% observer 2) and early repolarization abnormality (25% observer 1 and 14% observer 2).
The medical records of 941 patients undergoing coronary arteriography for presumed ACS within 48 h of onset were critically reviewed. In 70 patients (7.4%, 35 males) no CAD was documented. Alternative substrates of acute myocardial ischemia included coronary artery anomalies (7 patients, 10%), coronary spasm (10 patients, 14.3%), spontaneous coronary dissection (2 patients, 2.8%), paradoxical embolism through a patent foramen ovale (4 patients, 5.7%), embolism from left atrium or calcified aortic valve (4 patients, 5.7%), imbalance between oxygen demand and supply (20 patients, 28.5%), mitral valve prolapse (11 patients, 15.7%). No alternative substrates were found in 12 patients (17.1%). Absence of CAD is an uncommon finding in patients undergoing coronary artery angiography for ACS.