Written by Jesse McLaren
A 75 year-old patient with diabetes and end stage renal
disease was sent to the ED after dialysis for three days of nausea, vomiting,
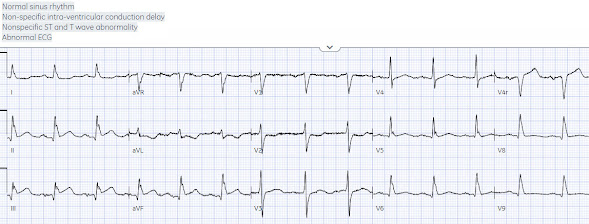
loose stool, lightheadedness and fatigue. RR18 sat 99% HR 90 BP 90/60, afebrile. Below is the 15 lead ECG. What do you think?
There’s normal sinus rhythm, normal conduction, normal axis,
normal R wave progression and normal voltages. There’s subtle inferior ST
elevation with straightening of the ST segment, reciprocal ST depression and T
wave inversion in aVL, and ST depression in V2. This is diagnostic of
infero-posterior OMI, but it is falsely negative by STEMI criteria and with
falsely negative posterior leads (though they do show mild ST elevation in V4R).
Because the patient had no chest pain or shortness of
breath, they were initially diagnosed as gastroenteritis. Potassium was normal. The ECG above was
only done an hour after arrival, when the first high sensitivity troponin I
returned at 300ng/L (normal <26 in males and <16 in females). But because
the patient had no chest pain or shortness of breath, it was not deemed to be
from ACS.
This is not unusual. In a large database of patients with
MI, those on dialysis had greater frequency of comorbidities but only 44%
presented with chest pain. They were less likely to have STEMI on ECG, and more
likely to be initially diagnosed as non-ACS. They had had twice the rate of
cardiac arrest and twice the in-hospital mortality[1]
In another study of patients diagnosed with
STEMI, those on dialysis experienced delayed reperfusion and double the
mortality.[2]
Two hours after arrival another ECG was done:
Now STEMI(+)OMI, but the patient still had no chest pain or
shortness of breath, so the ED physician requested a stat cardiology consult.
Cardiology did not think it was "STEMI", but repeated the troponin.
Four hours after arrival, the second troponin rose to 700,
and another 12 and 15 lead ECG were done:
Still OMI but not STEMI on anterior or posterior leads.
It would have been easy for this patient to be admitted as
“non-STEMI” with further delay to angiogram. Fortunately, the cath lab was activated:
99% proximal RCA occlusion with TIMI 2 flow. Peak troponin was 4,000 and echo
revealed basal/inferior wall hypokinesis.
Discharge ECG showed inferior Q waves and infero/posterior
reperfusion T waves:
ECG interpretation vs
clinical application
It’s common for the clinical picture to cloud ECG
interpretation. As with any test, the ECG is a tool for clinical
decision-making and needs clinical correlation. But the first step is correct
ECG interpretation, in isolation. You don’t need clinical assessment to know
the troponin because it has a specific value that doesn’t depend on patient
symptoms. The lab interprets the troponin in isolation, and then you correlate
the results clinically. It didn’t matter that the patient had no chest pain,
because their first troponin was 300; Similarly, it didn’t matter that the
patient had no chest pain, because their ECG showed OMI. But ECGs
interpretation is often conflated with clinical assessment, so if patients
don’t present with chest pain the ischemic changes are not seen or are attributed to something other than ischemia.
Instead, ECGs need to be interpreted in isolation and then
applied to the patient. This is how OMI can be taught to AI, which can help
identify patients at risk of missed occlusions. I sent the first ECG to the
Queen of Hearts app:
With
this high confidence on ECG interpretation in
isolation, the clinician could then apply this to the clinical picture: a
dialysis patient with GI symptoms and lightheadedness (potential anginal
equivalents), without any clinical evidence for this being a type 2 OMI,
and with myocarditis being a diagnosis of exclusion. This could have
expedited
cath lab activation and saved myocardium.
Take home
1.
Dialysis patients have a high rate of ACS
without chest pain and high rate of delayed diagnosis and delayed reperfusion
2.
Primary ST depression in aVL reciprocal to
subtle inferior ST elevation can identify inferior STEMI(-)OMI
3.
Ischemic ST depression max V1-4 is highly
specific for posterior OMI, and posterior leads can be falsely negative
4.
ECG interpretation needs to be done in isolation and then applied to the patient, and this way AI can help identify patients at risk of missed occlusions
References
1.
Herzog et al. Clinical characteristics of
dialysis patients with acute myocardial infarction in the United States. A
collaborative project of the United States Renal Data System and the National
Registry of Myocardial Infarction. Circulation 2007
2.
Khan et al. Contemporary trends and outcomes in
patients with ST-segment elevation myocardial infarction and end-stage renal
disease on dialysis: insights from the National Inpatient Sample. Cardiovasc
Resvasc Med 2020
===================================
MY Comment, by KEN GRAUER, MD (8/19/2023):
===================================
Today's case has similarities to other cases we frequently present in Dr. Smith's ECG Blog — namely despite clear signs on the initial ECG suggesting acute coronary occlusion — this stemi-negative but OMI-positive tracing was not only discounted after the initial ECG, but still not acted on even after the initial troponin assay came back elevated primarily because the patient did not have chest pain (See this December 6, 2022 case, also by Dr. McLaren — as well as others on Dr. Smith's ECG Blog). - In the hope of constructive commentary — I focus my observations on a number of Problems in Management that today's case illustrates — which can (and hopefully will) be corrected.
For clarity in Figure-1 — I've labeled today's initial tracing.
-USE%20copy.png) |
| Figure-1: The initial ECG in today’s case. |
Problem #1:
As I emphasized in My Comment in the December 6, 2022 post — Not all patients with acute MI report chest pain. The Framingham studies from many years ago taught us that the incidence of “Silent MI” is as high as ~30% of all MIs (Kannel & Abbott: N Engl J Med 311(18):1144-1147, 1984 — Kannel: Cardiol Clin 4(4):583-591, 1986). - The interesting part of this data is that in about half of this 30% (ie, ~15% of all patients with MI) — patients found on yearly follow-up ECGs to manifest clear evidence of infarction had NO symptoms at all — therefore truly “silent” MIs.
- But in the other half of this 30% (ie, in ~15% of all patients with MI) — although these patients found on follow-up ECG to have had infarction did not have chest pain — they did have "something else" thought to be associated with their MI.
- The most common “something else” symptom was shortness of breath. Other non-chest-pain equivalent symptoms included — abdominal pain — “flu-like” symptoms (ie, myalgias; not “feeling” good) — excessive fatigue — syncope — mental status changes (ie, as might be found in an elderly patient wandering from home).
- BOTTOM Line: Be aware of the entity of “Silent MI” — which can either be completely “silent” — or, associated with a non-chest-pain equivalent symptom. The incidence of both types of silent MI is more common than is often appreciated. We need to remember that not all patients with acute (or recent) MI have chest pain with their event.
- Application to Today's Case: As an older (ie, 75 years old) patient with diabetes (as well as end-stage renal failure) — who presented because of a several day history including GI symptoms, lightheadedness and fatigue, but no fever — today's patient had a host of features that might predispose to an infarction without chest pain.
Problem #2:
As should be evident in Figure-1 — Today's initial ECG is diagnostic of acute OMI.
- All 3 inferior leads not only demonstrate subtle-but-real ST elevation — but also hyperacute T waves (especially in lead III — in which there should be no doubt that the ST segment takeoff is overly straight — with a "fatter"-than-it-should-be T wave peak and, a wider-than-it-should-be ST-T wave base).
- In addition — infarction Q waves are seen in all 3 inferior leads (ie, Given modest QRS amplitude — each of these inferior Q waves is wider than I would expect for a septal or normal variant Q wave).
- The above said — the reason to emphasize that ECG #1 is diagnostic of acute OMI until you prove otherwise — is the mirror-image opposite (reciprocal) ST-T wave changes seen in lead aVL. Nothing else is needed — since these 4 leads definitively say, "Acute OMI until proven otherwise".
Problem #3:
Although no additional leads beyond leads II,III,aVF and aVL should be needed to justify (and activate) prompt cath — the remaining 9 leads on this initial 15-lead ECG provide more incontrovertible evidence:
- When doubt exists about the acuity of inferior OMI — ST-T wave changes in selected chest leads may suggest acute (or recent) posterior MI that reinforces an already decisive OMI diagnosis.
- As I've often emphasized — use of the Mirror Test facilitates rapid recognition of acute (or recent) posterior MI. This is based on the principle that ST depression maximal in leads V2-thru-V4 (especially when the shape of this ST depression is a mirror-image opposite of what acute stemi-elevation looks like) — strongly suggests posterior MI (See My Comment at the bottom of the page in the September 21, 2022 post).
- Chest lead ST-T wave findings in Figure-1 are admittedly subtle — but undeniably present. There normally is slight (usually 1-2 mm) upward-sloping ST elevation in anterior leads V2,V3. Not only do the BLUE arrows in these leads show a lack of this normal upward-sloping ST elevation — but the coved and depressed ST segment in lead V2 conveys a positive Mirror Test. Support to this diagnosis of presumed posterior MI is added by the abnormal J-point ST depression in lead V4.
- BOTTOM Line: Problem #3 is not using these subtle-but-real ST-T wave abnormalities in selected chest leads of Figure-1 to solidify the diagnosis of acute (or recent) inferior OMI.
Problem #4:
Even before we get to assessing the 3 additional leads in this 15-lead ECG — Problem #4 is the failure to consider acute RV involvement. After all — this elderly patient is predisposed to potentially having a "silent" MI with non-chest-pain symptoms as the presenting complaint.
- IF the inferior OMI that we've already diagnosed in today's case is associated with a significant component of acute RV involvement — then navigating optimal treatment in this elderly patient becomes that much more challenging (ie, This dialysis patient presented with 3 days of nausea, vomiting, diarrhea and now lightheadedness with fatigue — which if associated with volume-dependent acute RV involvement will make for a series of difficult-to-balance issues).
- Typically with acute infero-postero OMI in which we see hyperacute inferior lead ST-T waves in association with mirror-image opposite reciprocal changes as marked as we see in lead aVL — I would expect more ST depression than we see in leads V1-thru-V4. In particular in Figure-1 — right-sided lead V1 manifests no ST depression at all. So even before looking at lead V4R — I suspected acute proximal RCA occlusion with at least possible (if not probable) RV involvement.
- As good as it is that a 15-lead ECG was obtained as part of today's initial tracing (including right-sided lead V4R) — the fact that there is clear ST elevation in lead V4R (BLUE arrow in this lead highlighting J-point ST elevation) — was apparently discounted, again "because today's patient did not have chest pain".
Problem #5:
There apparently was dependence on posterior leads for the diagnosis of posterior infarction. As we've often emphasized in Dr. Smith's ECG Blog —
Posterior leads often provide false reassurance (See My Comment at the bottom of the page in the September 21, 2022 post). As occurred in today’s initial ECG —
despite our recognition of posterior OMI by the ST-T wave appearance in leads V2-thru-V4 in Figure-1 —
no ST elevation at all is seen in posterior leads V8,V9.
- Failure of posterior leads to consistently demonstrate ST elevation in association with subtle posterior OMI — should not be surprising. This is because posterior placement of leads V7, V8 and V9 situates these leads in a position from which electrical activity must pass through the thick musculature of the back before being recorded on the ECG. As a result — even under optimal circumstances, QRST amplitudes (and therefore the amount of ST-T wave elevation) in posterior leads is often modest.
- In contrast — because you are not having to traverse the thick back musculature to record a standard ECG (as you have to do when recording posterior leads) — the relative amplitude of ST-T wave segment deviations in anterior chest leads on a standard 12-lead ECG tends to be significantly larger than the ST-T wave amplitude seen with posterior leads. This is why I believe the Mirror Test is superior to use of posterior leads.
- EDITORIAL Note: I do not believe I have ever seen a case in which a posterior infarction diagnosed by posterior leads was not evident by use of the Mirror Test on the standard 12-lead ECG.

-USE.png)
-USE.png)
-USE.png)
-USE.png)








-USE%20copy.png)


-USE.png)