By the time we read the paper on the Barcelona rule, it was too late to write a letter to the editor or a commentary. So we are posting it here.
Here is the paper:
New Electrocardiographic Algorithm for the Diagnosis of Acute Myocardial Infarction in Patients With Left Bundle Branch Block
https://www.ahajournals.org/doi/10.1161/JAHA.119.015573
Diagnosis of Acute Occlusion Myocardial Infarction in the Setting of Left Bundle Branch Block: Thoughts on the Barcelona vs. the Modified Sgarbossa Rules
H. Pendell Meyers
Kenneth W. Dodd
Stephen W. Smith
Summary:
New rule was tested in patients referred for primary angioplasty (diagnosed with Occlusion MI by provider who referred the patient), compared to patients without any acute MI (neither OMI nor NOMI). The outcome of whether the patient actually had OMI was defined by culprit lesion plus minimally elevated troponin (above 1x the URL); this means that they were really comparing patients with any MI (OMI + NOMI) to patients without any MI at all. There was no differentiation between Non-OMI (equivalent to NSTEMI with an open artery) and No-MI (ruled out by negative troponins). The Modified Sgarbossa definition of OMI required either 1) TIMI 0/1 flow or 2) a culprit PLUS a very high troponin (200-300x the URL) which is the level seen in STEMI patients. The modified Sgarbossa rule differentiated between OMI and Non-OMI AMI, which Barcelona could not possibly do. There are more problems, outlined below.
Di Marco et al.1 performed a multicenter retrospective cohort study to derive and internally validate a new electrocardiographic algorithm known as the Barcelona algorithm for the ECG diagnosis of AMI in the setting of LBBB, and to compare the new algorithm against prior strategies including the original2 (OSC) and modified Sgarbossa criteria (MSC)3,4. For the diagnosis of any type 1 MI (equivalent in normal conduction to any NSTEMI or STEMI), they report the Barcelona algorithm to be superior to the MSC with higher sensitivity (93 vs. 68%, p<0.01) and equal specificity (94%). In this review we examine the compatibility of the results with prior data and discuss some major methodologic flaws of the study.
Results of Barcelona algorithm study compared to prior data
There are three differences between the Barcelona algorithm and the MSC:
1) While the MSC includes concordant ST depression (STD) in only leads V1-V3, the Barcelona algorithm considers concordant STD in any lead.
2) The MSC does not include excessively discordant STD, whereas the Barcelona criteria does.
3) For the definition of excessively discordant ST deviation (either ST elevation or STD), both strategies require at least 1.0 mm, but the MSC uses a quantified ratio of the discordant ST deviation to R or S wave amplitude of 20-25% in any lead, regardless of QRS voltage, whereas the Barcelona algorithm applies only to leads with maximum R/S voltage 6 mm and at least 1mm of ST deviation (meaning a minimum ratio of 1mm / 6mm = 17%).
If one of the two strategies is superior, then the improvement must originate from these three differences. Therefore it should be helpful to consider the prior data available for these differences.
1) Concordant STD in all 12 leads vs. V1-V3 only:
Concordant STD was studied in all 12 leads previously by Dodd and Smith.5 When the MSC (using concordant STD in V1-V3 only) were compared with an alternate version including concordant STD in any lead, the sensitivity of the rule remained unchanged (91%) while the specificity decreased (from 90% to 76%). Thus, the best prior evidence does not support extending concordant STD to all leads. A composite rule with combination of any concordance of at least 0.5 mm or ST/S ratio of at least 25% yielded 100% (95% CI 87–100) sensitivity and 57% (95% CI 47–65) specificity for the diagnosis of ACO in LBBB.
2) Excessively discordant STD
Excessively discordant STD in LBBB was first studied in the derivation study3 of the MSC by Smith et al, in which the optimal cutpoint for discordant ST deviation was calculated to be 30% (STD of at least 30% of the preceding R wave), which produced sensitivity and specificity of 100% and 88% for acute coronary occlusion myocardial infarction (OMI, not for any AMI). Meyers and Smith replicated the study design in a validation study4 and found lower sensitivity of only 64%, but with 98% specificity. Thus, although the specificity was even higher in this separate validation sample by the same author group, the sensitivity of excessively discordant STD was not replicated, and it was therefore not incorporated into the MSC. The new data from Di Marco et al. offer renewed interest in the role of excessively discordant STD in future validation studies.
3) MSC’s 20-25% ratio for excessive discordance vs. Barcelona algorithm’s 1 mm ST deviation in leads with R/S voltage 6 mm
The Barcelona algorithm uses a ratio of 1 mm / 6 mm = 17%, similar to the MSC’s lower studied ratio of 20%. However, the Barcelona algorithm’s ratio is not applied in leads with > 6mm maximum R/S component. Thus, we do not know what percent of patients in this algorithm require concordant STE to be positive. It is difficult to fathom how excluding all patients with excessive discordance who have an R- or S-wave greater than 6 mm could improve sensitivity.
For example Figure 1 below shows the ECG of a patient with 100% acute thrombotic LAD occlusion. The ECG has no concordant STD or STE, and is positive by the MSC due to excessively discordant STE (of > 25%) in V2, V3, and V4. However, the ECG contains no leads with maximum R or S wave 6 mm or less (other than aVR), and therefore is a false negative by the Barcelona algorithm (aVR has a 2mm R wave and a 2 mm S wave, with < 1 mm ST deviation).
Figure 2 shows the ECG of another patient with LBBB and an acute TIMI-0 LAD occlusion. There is no concordant STE or STD. There is discordant STE in V1-V5 which meets the MSC criterion in all 5 consecutive leads, but meets the Barcelona algorithm criterion only in lead V5, because V1-V4 have predominant S waves which are greater than 6 mm in amplitude, whereas the S wave in V5 is exactly 6 mm. Thus, a realistic and imperfect practitioner has five consecutive leads to identify using the MSC versus only one lead using the Barcelona criteria (assuming the practitioner measures the S wave perfectly and does not exclude the lead due to a perceived S wave of 7.0 mm). For these reasons, it is unclear how these new criteria could improve the ECG diagnosis of OMI in LBBB.
Figure 1
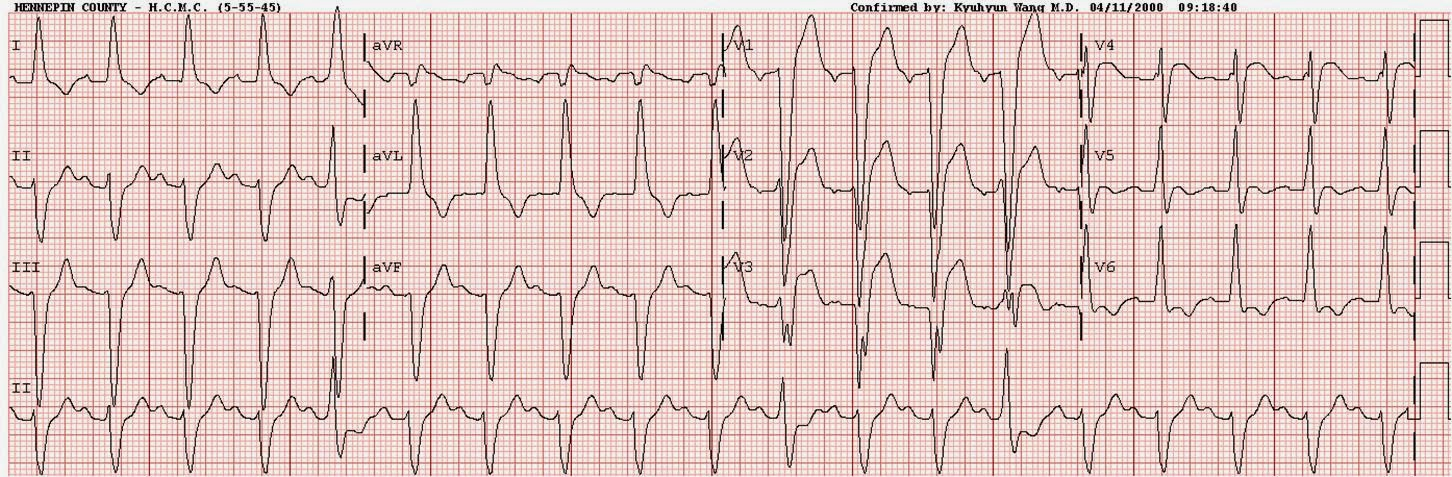
Figure 2

It is hard to imagine why limiting proportionally excessively discordant ST Elevation only to leads with an R- or S-wave of less than 6 mm could improve the rule. In both our derivation and validation studies of the MSC, many cases were only identified by a high ST/S ratio in leads with large S-waves. The answer lies most likely in the methodologic flaws outlined below.
Methodologic flaws of Di Marco et al.
Patient Population
Unlike the derivation and validation studies of the MSC which used a control group of ED patients with potential ischemic symptoms, Di Marco et al. used a control population of patients with no clinical concern for ACS, which likely overestimates the specificity of all strategies studied. Furthermore, all case patients (non-control patients) used in Di Marco were identified by referral for primary PCI, which does not appropriately represent the entire population of ED patients with LBBB and possible ACS. Thus, cases have an extremely high, and controls an extremely low, pretest probability, which, contrary to conventional wisdom, does affect sensitivity and specificity.6
Outcome Definitions
In contrast to the MSC studies, which used angiographic occlusion (diagnosed by TIMI 0/1 or any culprit withy very high troponin) as the primary outcome, the primary outcome used in Di Marco et al., was “AMI” with any culprit lesion of any TIMI flow score and any rise and/or fall of troponin above the upper reference limit (essentially, any type 1 MI, equivalent in normal conduction to all type 1 STEMI and all type 1 NSTEMI).
Although the Barcelona algorithm appears to show very high sensitivity for any type 1 MI (both STEMI and NonSTEMI), all previous studies of ST Elevation in normal conduction (no LBBB) have shown much lower sensitivity for any type 1 MI. For example, among a prospective, real world population of 2486 patients in the ED with ACS symptoms, of whom 438 had type 1 MI, Hillinger et. al. reported sensitivity of only 17% for any Type 1 MI (STEMI or NonSTEMI) in normal conduction, with cardiologists using formal STEMI criteria7. This is not surprising because the ECG is known to be very insensitive for any MIs, no matter whether in LBBB or normal conduction. Since the ECG is so insensitive, and troponin can be used to diagnose MI that does not need the cath lab emergently, the current role of the ECG is to diagnose OMI, which needs emergent reperfusion. The fact that the Barcelona algorithm was found to have 93 and 94% sensitivity and specificity for any type 1 MI in LBBB is therefore impossible to reconcile with previous data on the ECG in the diagnosis of AMI. Thus, Di Marco’s primary outcome definition was not an appropriate or reasonable goal to ask of the ECG, and it is unclear how such a high degree of accuracy was possible for this outcome.
Even if the ECG could accurately predict any MI, the standard management of patients with NSTEMI who are pain free and without OMI is to wait up to 36 hours for angiography and PCI. Thus, Di Marco’s outcome definition including TIMI 3 flow and minimal troponin rise and fall is unlikely to represent patients who require emergent reperfusion. While many STEMIs do not have complete occlusion (TIMI 0) at the time of angiography, the majority have large troponin elevations.8 For this reason, the MSC studies used full occlusion (TIMI 0/1 flow) but also used a surrogate endpoint for an acute coronary occlusion that may have been present at the time of the ECG, but not at the time of angiography: any culprit, even with TIMI 2-3 flow if there was also a “highly elevated” troponin defined as troponin I >10.0 ng/mL or troponin T > 1.0 ng/mL.
In their supplemental information, Di Marco et al. provide a secondary outcome definition which they state was modeled after the outcome definition used in the MSC studies, however the troponin cutoffs used are markedly different. Di Marco et al. used a troponin ratio (peak level divided by upper reference limit) of 10, referencing Gonzalez et al.9 Moreover, this is a mistaken representation of Gonzalez et al. who instead stated that 11.3% of STEMI patients had a peak troponin I of 7.1 ng/mL. Moreover, Gonzalez et al. erroneously state that their troponin assay has an 99% URL of >0.001 µg/L, a critical mistake: there is no assay on the market which has a URL of 0.001 µg/L. Using this URL, a peak troponin ratio of 10 gives a cutpoint of 0.010 ng/mL, which is not only a very low cutoff for occlusion, it is too low even to make the diagnosis of MI, as it lower than the URL for almost all assays.
In the MSC validation study, by comparison, the vast majority of troponin I measurements were done using an assay with URL of 0.032 to 0.050 ng/mL (µg/L). Thus, the troponin I ratio in our study was 10.0 / 0.05 ng/mL (µg/L) = 200, far greater than 10 as in Di Marco et al. Similarly, the most appropriate equivalent troponin T cutpoint is estimated at 1.0 ng/mL.10 The proven TIMI 0-1 Occlusion MI groups in our studies consistently show mean peak troponin T levels in the 2.0-6.0 ng/mL (µg/L) range, despite the fact that the two sites have assays with a 10-fold difference in the URL (0.01 vs. 0.10 ng/mL). This highlights the inappropriateness of fixed troponin ratios for comparing the peak troponin levels in STEMI and Occlusion MI among assays with vastly different URLs.
As expected with the inappropriately low troponin threshold in Di Marco et al, the online supplemental information reveals that 95% of all AMIs in the study were considered “STEMI equivalents.” Obviously not all AMIs should be considered “STEMI equivalents”, and this is not in keeping with the known prevalence of true STEMI among AMI in normal conduction. For example, Hillinger et al. found only 81 STEMIs (18%) and 135 Occlusion MI (31%) among 438 AMIs in their large, prospective, real world chest pain cohort.7
Conclusion
Despite the profound methodological flaws, there may be utility of some components of the Barcelona algorithm vs. those of the MSC. If such individual components are confirmed by external validation studies, perhaps a rule with better overall performance could be formulated. Most importantly, we must understand that no ECG rule will likely ever identify all AMI in either LBBB or normal conduction, and so seek to maximize the potential of the ECG to identify Occlusion MI.
References:
1. Di Marco, A. et al. New Electrocardiographic Algorithm for the Diagnosis of Acute Myocardial Infarction in Patients With Left Bundle Branch Block. J. Am. Heart Assoc. e015573 (2020) doi:10.1161/JAHA.119.015573.
2. Sgarbossa, E. B. et al. Electrocardiographic Diagnosis of Evolving Acute Myocardial Infarction in the Presence of Left Bundle-Branch Block. N. Engl. J. Med. 334, 481–487 (1996).
3. Smith, S. W., Dodd, K. W., Henry, T. D., Dvorak, D. M. & Pearce, L. A. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann. Emerg. Med. 60, 766–776 (2012).
4. Meyers, H. P. et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: A retrospective case-control study. Am. Heart J. 170, 1255–1264 (2015).
5. Dodd, K. W., Elm, K. D. & Smith, S. W. Comparison of the QRS Complex, ST-Segment, and T-Wave Among Patients with Left Bundle Branch Block with and without Acute Myocardial Infarction. J. Emerg. Med. 51, 1–8 (2016).
6. Leeflang, M. M. G., Rutjes, A. W. S., Reitsma, J. B., Hooft, L. & Bossuyt, P. M. M. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 185, E537–44 (2013).
7. Hillinger, P. et al. Prospective validation of current quantitative electrocardiographic criteria for ST-elevation myocardial infarction. Int. J. Cardiol. (2019) doi:10.1016/j.ijcard.2019.04.041.
8. Karwowski, J. et al. Total coronary occlusion of infarct-related arteries in patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary revascularisation. Kardiol. Pol. 75, 108–116 (2017).
9. Gonzalez, M. A. et al. Quartiles of Peak Troponin Are Associated with Long-term Risk of Death in Type 1 and STEMI, but Not in Type 2 or NSTEMI Patients. Clinical Cardiology vol. 32 575–583 (2009).
10. Baro, R., Haseeb, S., Ordoñez, S. & Costabel, J. P. High-sensitivity cardiac troponin T as a predictor of acute Total occlusion in patients with non-ST-segment elevation acute coronary syndrome. Clin. Cardiol. 42, 222–226 (2019).






