This case was sent by David Carroll, a 2nd year EM resident, and his attending physician Brad Caloia.
A 60-something male presented to the ED with weakness and fatigue. He was diagnosed with a viral syndrome and discharged.
He returned later and had a lab and ECG workup. He had no cardiac history. There was no chest pain or shortness of breath.
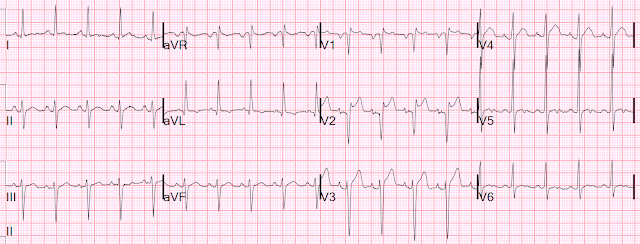
Here is his ECG:
Dr. Carroll astutely realized something was amiss: what is it?
There is no ST Segment (i.e.,the ST segment is extremely short). The T-wave follows immediately after the end of the QRS. There is also a domed T-wave. Both of these are typical of hypercalcemia, and Dr. Carroll recognized this. However, usually the short ST results in a short QT also. But in this case the QT is accurately measured by the computer. The domed T-wave mimics STEMI.
Being a careful clinician, while awaiting the result of blood tests, he recorded another ECG 33 minutes later:
The calcium returned at 20.0 mg/dL (twice normal). He began therapy for hyperCa and obtained a chest X-ray:
Metastatic cancer
6 hours later, the Ca was down to 14.5 and another ECG was recorded:
The ST segment is still short. The T-waves are less domed. But the QT is the same.
Here are 2 more examples of hypercalcemia, both with short QT and short ST segment:
This is from K.Wang's book (V1-V6 only):
How are these cases related?
Learning Points:
1. Hypercalcemia shortens the ST segment
2. The QT can be normal in the setting of a short ST segment if the remainder of the QT is long
===================================
MY Comment by KEN GRAUER, MD (10/30/2020):
===================================
I thought today’s case provided a wonderful example of an important ECG finding that often goes unrecognized. CREDIT to Dr. David Carroll — who quickly picked up on this finding!
- Although 3 serial ECGs are presented in above in this case — I did not think there was a significant difference between them. As a result — I focus my comments on the initial ECG, which I’ve reproduced in Figure-1.
MY Thoughts regarding ECG #1:
As we emphasize repeatedly — the History is critical (!) for optimal clinical ECG interpretation. The presenting complaint of the 60-something man whose ECG is shown in Figure-1 was weakness and fatigue — but not chest pain!
- Although I’ve drawn attention on a number of occasions to the entity of “silent MI”, in which acute OMI may occur in the absence of chest pain — the history in today’s case should preferentially heighten awareness of a possible non-cardiac cause for this patient’s weakness and fatigue.
- As to the ECG itself — the rhythm in ECG #1 is sinus at a rate of ~85/minute. All intervals (PR, QRS, QTc) and the axis are normal. There is no chamber enlargement.
Regarding Q-R-S-T Changes in ECG #1:
- There are no Q waves (other than in lead aVR, which is not clinically significant).
- R wave progression is normal (with transition where the R wave becomes taller than the S wave is deep occurring normally, between leads V3-to-V4). S waves persist through to lead V6.
- There is ST segment coving in leads V2-thru-V6. There appears to be some ST elevation in each of these leads — though quantification of the amount of J-point ST elevation is difficult to do because of how smooth the ST coving is. There is non-specific ST-T wave flattening in each of the limb leads.
Putting It All Together: Regarding my Clinical IMPRESSION of the above noted ECG findings in ECG #1 — I would highlight the following:
- PEARL #1 — There is significant baseline artifact in the limb leads of ECG #1. As I’ve noted previously — the EASY way to quickly identify the “culprit” extremity causing the artifact is to see IF artifact is maximal in 2 of the limb leads, and in 1 of the augmented leads. According to Einthoven’s Triangle — since the artifact in ECG #1 is maximal in limb leads I and II — and in augmented lead aVR — the “culprit” extremity is most probably due to a problem (ie, tremor) in the RA ( = Right Arm).
- NOTE: For detailed description on HOW to identify the “culprit extremity” causing artifact — Please SEE My Comment at the bottom of the page in our September 27, 2019 post in Dr. Smith’s ECG Blog.
PEARL #2: As per Dr. Smith’s calculations above — the QTc interval is normal in ECG #1.
- I measure a QT interval of 350 msec in several of the chest leads, which given the heart rate of ~85/minute — I estimated a QT corrected-for-rate of ~420 msec ( = clearly within the normal range).
- That said, despite this normal QTc value — the QT interval “looks” short! As noted above by Dr. Smith — the reason the QTc looks short, is that there is virtually no ST segment in ECG #1.
- While ST coving in association with J-point ST elevation in a number of consecutive chest leads can clearly be the result of acute ischemia — We need to remember that the differential diagnosis of a short QTc (or a QTc that looks short) is limited = short QT syndromes and/or hypercalcemia.
- It also helps to remember that the QTc tends to be longer with acute ischemic heart disease. Lack of a longer QTc in ECG #1 supports a non-ischemic etiology.
Hypercalcemia to at least a moderate degree (ie, serum calcium level >12 mg/dL) is not a common diagnosis in an unselected ED population. Instead, the prevalence of this electrolyte disorder will be much higher in an oncology population (ie, more than 90% of patients with hypercalcemia have either primary hyperparathyroidism or malignancy).
- This is where the History in today’s case comes in — as this 60-something man presented with weakness and fatigue, but no chest pain (ie, Malignancy rises as a diagnostic consideration).
- PEARL #3: While the textbook description of ECG findings of hypercalcemia is often limited to “QT interval shortening” — QT shortening is not an easy ECG finding to recognize (even when you are looking for it!). In addition, what is not described in textbooks — is how high the serum Ca++ must go before such QT interval shortening occurs. Over my 3 decades as family medicine Attending (working in and out of the hospital) — I religiously scrutinized the ECGs of all patients I encountered in whom serum calcium levels were elevated. In my experience — NO change in ECG appearance was noted in the overwhelming majority of hypercalcemic patients until their serum Ca++ level was significantly elevated (ie, generally over 12 mg/dL). Therefore — Do not expect to pick up hypercalcemia on ECG unless serum Ca++ is increased by a lot.
- PEARL #4: More than simply QT interval “shortening” — the principal ECG finding of significant hypercalcemia is a short-Q-to-peak-of-T interval. By this I mean that the time it takes for the T wave to attain its peak is shortened with significant hypercalcemia. I know of no measurement to quantify this shortened time-until-T-wave-peak. Instead — it is a subjective judgment — that with experience (armed by an increased index of suspicion for the case-at-hand) YOU can learn to appreciate.
- Regarding ECG #1 — I found it extremely difficult to appreciate the time-until-T-wave-peak in this tracing because the coved ST segment is so smooth in most chest leads. I did draw in vertical BLUE lines in leads V4 and V5 of ECG #1 at the point in these leads where I thought definite “peaking” was seen. Subjectively — the time until attaining this T wave peak seemed short to me with respect to the vertical RED line that marks the end of the T wave in these leads.
CASE Continuation: As discussed above — the serum Ca++ level obtained at the time ECG #1 was recorded, was dramatically increased to 20.0 mg/dL. Diffuse metastatic cancer was obvious on chest X-ray.
- As I noted earlier — I did not see significant change in the 2 ECGs shown above that were obtained after ECG #1 (despite the fact that serum Ca++ had decreased significantly to 14.5 mg/dL by the time the 3rd ECG shown above was obtained).
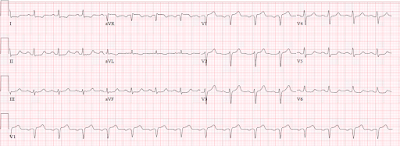
PEARL #5: To better illustrate what I mean by a short-Q-to-peak-of-T interval — I’ve reproduced an ECG from My Comment at the bottom of the page in the July 1, 2020 post in Dr. Smith’s ECG Blog (Figure-2).
- Once again — the History in this July 1, 2020 case was insightful. My years in primary care taught me to be leery of older patients presenting with an unusual history of atypical pain in some specific part of their body. While clearly patients with “frozen shoulder” may experience exacerbations in the degree of their shoulder pain — I immediately became suspicious after hearing this history and seeing the ECG in Figure-2, that the patient had hypercalcemia secondary to malignancy. The remarkable ECG finding in this tracing is a short QTc interval.
- Vertical BLUE lines in leads V2 and V3 of this ECG from July 1, 2020 are placed over the peak of the T waves in these leads. Doesn’t the time until attaining this T wave peak “look” short? The serum Ca++ level corresponding to this ECG was 15 mg/dL — and the patient was found to have lung cancer with shoulder metastases accounting for the increase in his extremity pain.
- Now look at the Inserts in leads V2 and V3 in Figure-2. I’ve placed one QRST complex within each insert from the repeat ECG after correction of the elevated serum Ca++ level. Doesn’t the time until T wave peaking (marked by vertical GREEN lines within the inserts) now look longer (and more normal) after correction of the serum Ca++ level?
- BOTTOM Line ( = My Synthesis): I have not found much literature regarding clinical correlation between various serum Ca++ levels and time-until-T-wave peaking. Despite as high of a serum Ca++ level ( = 20 mg/dL) as you will probably ever see for the patient in today’s case — and despite a QT interval that “looks” short in ECG #1 — the QTc (corrected for rate) for the ECG in Figure-1 was not short. Significant reduction in the serum Ca++ level obtained 6 hours later did not produce appreciable change in ECG appearance. In contrast — the ECG in Figure-2 did show subtle-but-real ECG changes of hypercalcemia that did improve once serum Ca++ levels returned to normal. My "Take” — Each patient is different, and even with experience it is challenging to appreciate subtle ECG changes in many patients with hypercalcemia.
- Final PEARL: Awareness of the clinical history often provides invaluable assistance when interpreting tracings such as the ECGs shown in Figures 1 and 2.
Figure-2: This ECG is reproduced from My Comment in the July 1, 2020 post in Dr. Smith’s ECG Blog (See text).
P.S. — For another example of ST elevation from hypercalcemia — CLICK HERE —
ADDENDUM (11/2/2020):
Credit to Praneet Manekar — who picked up on an additional finding associated with hypercalcemia. I would be remiss not to add this to My Comment. As I’ve indicated on a number of occasions — although Osborn waves are most commonly associated with hypothermia — they can on occasion be seen with other conditions (SEE My Comment at the bottom of the page in the November 22, 2019 post in Dr. Smith’s ECG Blog).
- The Osborn wave is described as a deflection with a dome or hump, that occurs at the point where the end of the QRS complex joins with the beginning of the ST segment. This is the J-Point (ie, it Joins the end of the QRS with the beginning of the ST segment) — so Osborn waves are exaggerated J-point waves.
- In addition to hypothermia — Osborn waves have been reported with brain inury, subarachnoid hemorrhage, Brugada syndrome, cardiac arrest from VFib, severe ischemia — and, as in the above case, hypercalcemia (RED arrows in the inferior leads of ECG #1 — as shown in Figure-3).
- I would have loved to see a follow-up ECG on this patient after serum Ca++ had returned to normal — in order to see if these inferior lead Osborn waves resolved. (Otero & Lenihan show resolution of hypercalcemia-induced Osborn waves after correction of serum Ca++ in this case report — Tex Heart Inst J 27:316, 2000 ).
- My THANKS again to Praneet Manekar.