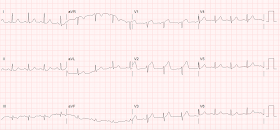
I was shown this ECG of a patient with dyspnea:
 |
My interpretation: "Subacute STEMI"
In other words, STEMI of at least 6 hours duration, and more typically greater than 12 hours. |
ECG differential may include: Old anterior MI with persistent ST Elevation (
LV aneurysm morphology).
Which is it?
My rule for differentiating acute STEMI from LV aneurysm really only reliably distinguishes between:
1. acute STEMI on the one hand
2. subacute STEMI or LV aneurysm on the other.
What is the rule?
First, there must be ST Elevation.
Second, the ECG differential diagnosis much be LV aneurysm vs. acute STEMI.
This rule should not be used for
early repol vs. acute STEMI. Conversely, if the differential is LV aneurysm vs. acute STEMI, then you should NOT use the
early repol formula.
When should LV aneurysm be on the ECG differential diagnosis? Primarily when there are well-formed Q-waves, with at least one QS-wave, in V1-V4. A QS-wave is defined by absence of any R-wave or r-wave of at least 1 mm. (If there is an R-wave or r-wave, we call the whole wave a QR-wave, Qr-wave, or qR-wave, depending on the relative size of the Q-wave vs. R-wave.)
The rule: If there is one lead of V1-V4 in which the T/QRS ratio is greater than 0.36, then acute STEMI is the likely diagnosis, though subacute STEMI is also possible. Since both require the cath lab, if the ratio is greater than 0.36,
and the clinical situation is right (i.e., unexplained chest discomfort), then cath lab activation is indicated. I both derived and validated this formula, for which the cutoff has good sensitivity and specificity:
Derivation:
http://www.sciencedirect.com/science/article/pii/S0735675705000811
(accuracy of formula = 93.2%)
Validation:
http://www.sciencedirect.com/science/article/pii/S0735675715001904
Validation full text
Sensitivity 91%, specificity 81%
False negatives had pain duration greater than 6 hours. Thus, it may classify those patients with prolonged chest pain as LV aneurysm when they are really subacute STEMI.
This case
If the formula were used here, then lead V4 would have a T/QRS ratio of 5/11.5 = 0.43. Only one lead is needed,
so the criterion is met for acute STEMI.
Really, however, LV aneurysm was never on my differential diagnosis when I glanced at this ECG.
Why? It is true that there are QS-waves in all of V1-V4, which might lead you to believe it is LV aneurysm. However, this ECG has up to 4 mm of ST elevation (in V4, 4 mm STE is relative to an 11.5 mm S-wave), and
I have never seen an LV aneurysm case with this much ST elevation.
This is a typical LV aneurysm:
Then my partner told me about the case:
A middle aged woman presented in pulmonary edema. She had suffered from chest pain 2 weeks earlier.
She had an immediate bedside ultrasound:
Many B-lines of pulmonary edema
Here is a view of her left ventricle:
Akinetic Septum and Apex. Echo differential is large anteroseptal MI vs. Takotsubo.
Short axis view:
Akinetic septum. Since this is more focal, takotsubo is much less likely.
Here is a view using Speckle tracking strain echocardiography, which can be done on our ED POCUS machines.
There is a surprising amount of collapse of the inferior vena cave (IVC):
She was stabilized on noninvasive positive pressure ventilation and was able to lie flat for an angiogram.
The patient was taken to the cath lab and a 100% thrombotic mid-LAD occlusion was found.
Troponin I profile:
 |
| This troponin profile is consistent with an MI that occurred many days ago and was much higher then. The slight rise may be due to the additional stress of acute pulmonary edema. |
Echo on day 3:
38% ejection fraction
Anteroapical Wall motion abnormality.
ECG:
 |
Now the T/QRS ratio is 4/13,which is less than 0.36
But it is more than it should be after successful reperfusion.
This is a bad sign and indicates poor microvascular reperfusion.
Such persistent ST elevation after reperfusion is an ECG sign that an LV aneurysm will develop. |