A young woman presented with substernal chest pain described as both crushing and stabbing, with radiation to the jaw and left arm and associated with dyspnea. It was not positional or pleuritic. She was otherwise healthy except for an unspecified "recent illness" and recent pyelonephritis. She denied tobacco or other drug use.
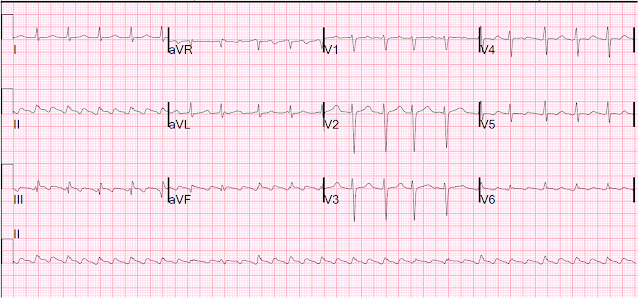
Here is her initial ECG:
We have found that, in inferior STEMI, there is always some, at least minimal, amount of reciprocal ST depression in aVL (manuscript being submitted). Of course, if this is inferior STEMI, it is really inferolateral STEMI. Does inferolateral STEMI also have reciprocal ST depression in aVL? In my experience, yes. I have yet to see an inferolateral STEMI without some reciprocal ST depression in aVL, in spite of the lateral ST elevation in V5 and V6. We will be re-analyzing our data to look at that.
Value of ST elevation in lead II vs. lead III for diagnosis of inferior MI vs. non-MI etiologies of "inferior" ST elevation:
A lot of attention is given to whether the ST elevation in lead II is greater than, or equal to, that in lead III: many claim that, if it is, then it is not inferior STEMI, but rather pericarditis. First, it is important to point out that baseline inferior ST elevation (early repol of the inferior leads) is more common than pericarditis, and if a patient complains of chest pain, and happens to have baseline inferior early repol, they are likely to get a diagnosis of pericarditis if they rule out for ACS. In our research (not yet published), we found that STE in lead II was greater than or equal to STE in lead III in 49 of 49 cases of pericarditis, but only 32 of 66 cases of early repol. Thus, in non-MI etiologies of inferior ST elevation, STE in lead II was greater than or equal to STE in lead III in only 81/115 cases. In inferior MI, STE in lead II was greater than or equal to STE in lead III in only 6 of 155 cases. Thus, the sensitivity for inferior MI of lead III greater than or equal to Lead II STE elevation was high, at 96%, but it had only 70% specificity. This does not compare favorably with any ST depression in aVL, which had 99% sensitivity and specificity. Thus, do not compare STE in lead II and III; use STD in aVL only.
Clinical Course
The ED physician ordered another ECG and it was unchanged. He also performed a bedside ultrasound and saw no effusion and could not identify any wall motion abnormalities. A third ECG (not shown) looked improved. The initial troponin I returned at 6.1 ng/mL and so, with a dynamic ECG and positive troponin, the cath lab was activated.
The angiogram was normal. A formal Echo the next AM was normal, with no effusion and no wall motion abnormality. Although no rub was ever heard, and she did not undergo MRI, she was diagnosed with myocarditis. She had had fevers and chills and myalgias, and a CRP mildly elevated.
Sarda et al. (free full text) studied patients with apparent MI but normal angiograms ("apparent MI" means they excluded patients who clearly had clinical myocarditis) and investigated them with Indium-111 Scintigraphy antimyosin antibodies, allowing noninvasive diagnosis of myocarditis. In 2 patients, they found MI, presumably due to reperfused occlusion or to vasospasm. In 17, diffuse myocarditis was found, and in 18, focal myocarditis was found, and 8 had no tracer uptake. Thus, 35 of 45 cases were myocarditis. Among all these patients, only 2 of 35 had any reciprocal ST elevation anywhere on the ECG even though 22 of 35 had regional wall motion abnormalities.
Thus, reciprocal ST depression in aVL is very sensitive and specific for MI, and very unusual in myo-pericarditis.
This is a scary ECG and activating the cath lab is certainly not wrong. However, analysis of aVL is very accurate in predicting an non-MI etiology of this ST elevation, and if an emergent high quality echocardiogram can be done while the ECG has ST segment elevation, it could rule out STEMI and save this patient a trip to the cath lab on an emergent basis. (If done after resolution of ST elevation, there could be reperfusion with recovery of wall motion, although recovery of wall motion does not often recover immediately.)
Here is her initial ECG:
We have found that, in inferior STEMI, there is always some, at least minimal, amount of reciprocal ST depression in aVL (manuscript being submitted). Of course, if this is inferior STEMI, it is really inferolateral STEMI. Does inferolateral STEMI also have reciprocal ST depression in aVL? In my experience, yes. I have yet to see an inferolateral STEMI without some reciprocal ST depression in aVL, in spite of the lateral ST elevation in V5 and V6. We will be re-analyzing our data to look at that.
Value of ST elevation in lead II vs. lead III for diagnosis of inferior MI vs. non-MI etiologies of "inferior" ST elevation:
A lot of attention is given to whether the ST elevation in lead II is greater than, or equal to, that in lead III: many claim that, if it is, then it is not inferior STEMI, but rather pericarditis. First, it is important to point out that baseline inferior ST elevation (early repol of the inferior leads) is more common than pericarditis, and if a patient complains of chest pain, and happens to have baseline inferior early repol, they are likely to get a diagnosis of pericarditis if they rule out for ACS. In our research (not yet published), we found that STE in lead II was greater than or equal to STE in lead III in 49 of 49 cases of pericarditis, but only 32 of 66 cases of early repol. Thus, in non-MI etiologies of inferior ST elevation, STE in lead II was greater than or equal to STE in lead III in only 81/115 cases. In inferior MI, STE in lead II was greater than or equal to STE in lead III in only 6 of 155 cases. Thus, the sensitivity for inferior MI of lead III greater than or equal to Lead II STE elevation was high, at 96%, but it had only 70% specificity. This does not compare favorably with any ST depression in aVL, which had 99% sensitivity and specificity. Thus, do not compare STE in lead II and III; use STD in aVL only.
Clinical Course
The ED physician ordered another ECG and it was unchanged. He also performed a bedside ultrasound and saw no effusion and could not identify any wall motion abnormalities. A third ECG (not shown) looked improved. The initial troponin I returned at 6.1 ng/mL and so, with a dynamic ECG and positive troponin, the cath lab was activated.
The angiogram was normal. A formal Echo the next AM was normal, with no effusion and no wall motion abnormality. Although no rub was ever heard, and she did not undergo MRI, she was diagnosed with myocarditis. She had had fevers and chills and myalgias, and a CRP mildly elevated.
Sarda et al. (free full text) studied patients with apparent MI but normal angiograms ("apparent MI" means they excluded patients who clearly had clinical myocarditis) and investigated them with Indium-111 Scintigraphy antimyosin antibodies, allowing noninvasive diagnosis of myocarditis. In 2 patients, they found MI, presumably due to reperfused occlusion or to vasospasm. In 17, diffuse myocarditis was found, and in 18, focal myocarditis was found, and 8 had no tracer uptake. Thus, 35 of 45 cases were myocarditis. Among all these patients, only 2 of 35 had any reciprocal ST elevation anywhere on the ECG even though 22 of 35 had regional wall motion abnormalities.
Thus, reciprocal ST depression in aVL is very sensitive and specific for MI, and very unusual in myo-pericarditis.
This is a scary ECG and activating the cath lab is certainly not wrong. However, analysis of aVL is very accurate in predicting an non-MI etiology of this ST elevation, and if an emergent high quality echocardiogram can be done while the ECG has ST segment elevation, it could rule out STEMI and save this patient a trip to the cath lab on an emergent basis. (If done after resolution of ST elevation, there could be reperfusion with recovery of wall motion, although recovery of wall motion does not often recover immediately.)